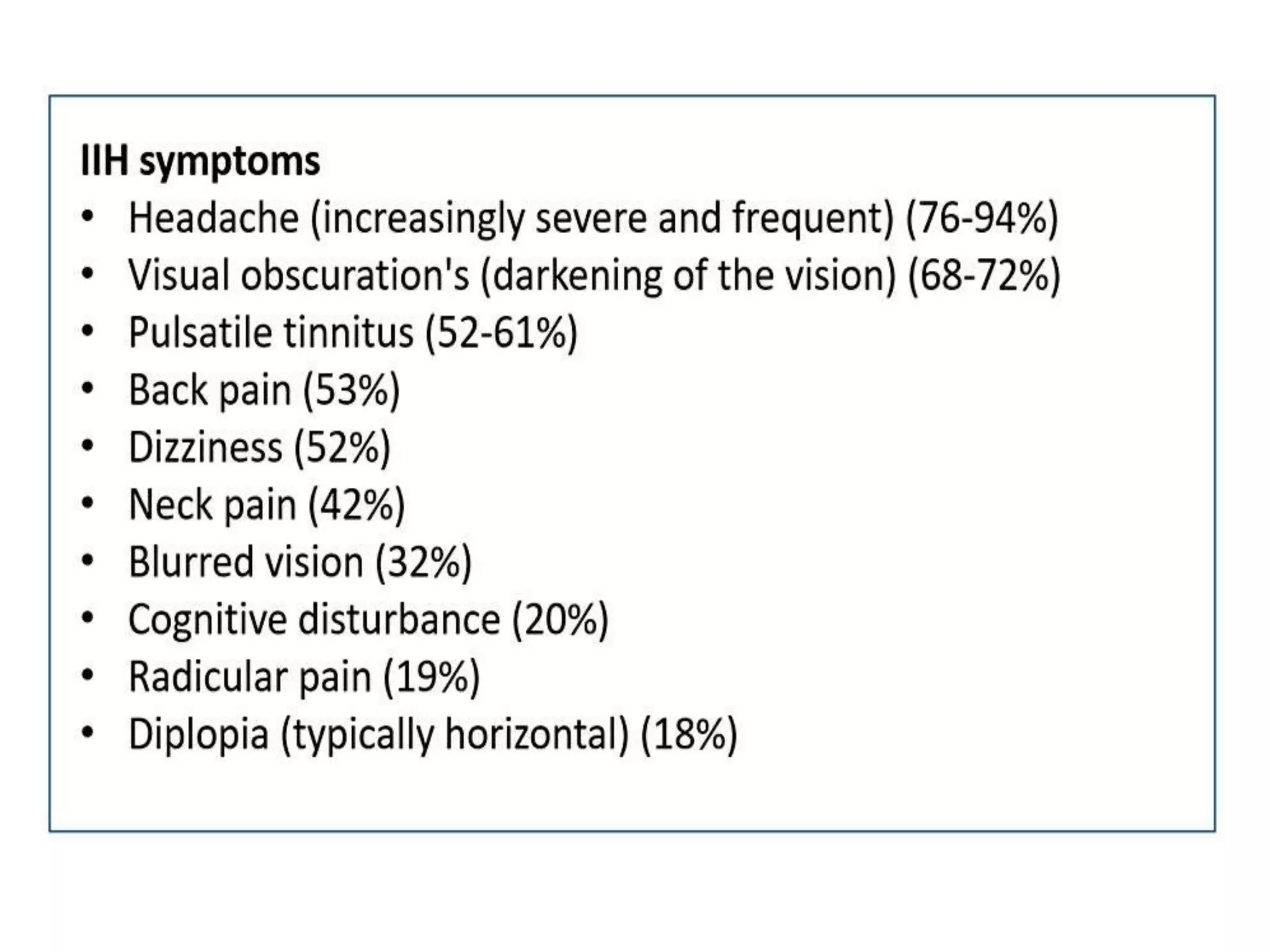
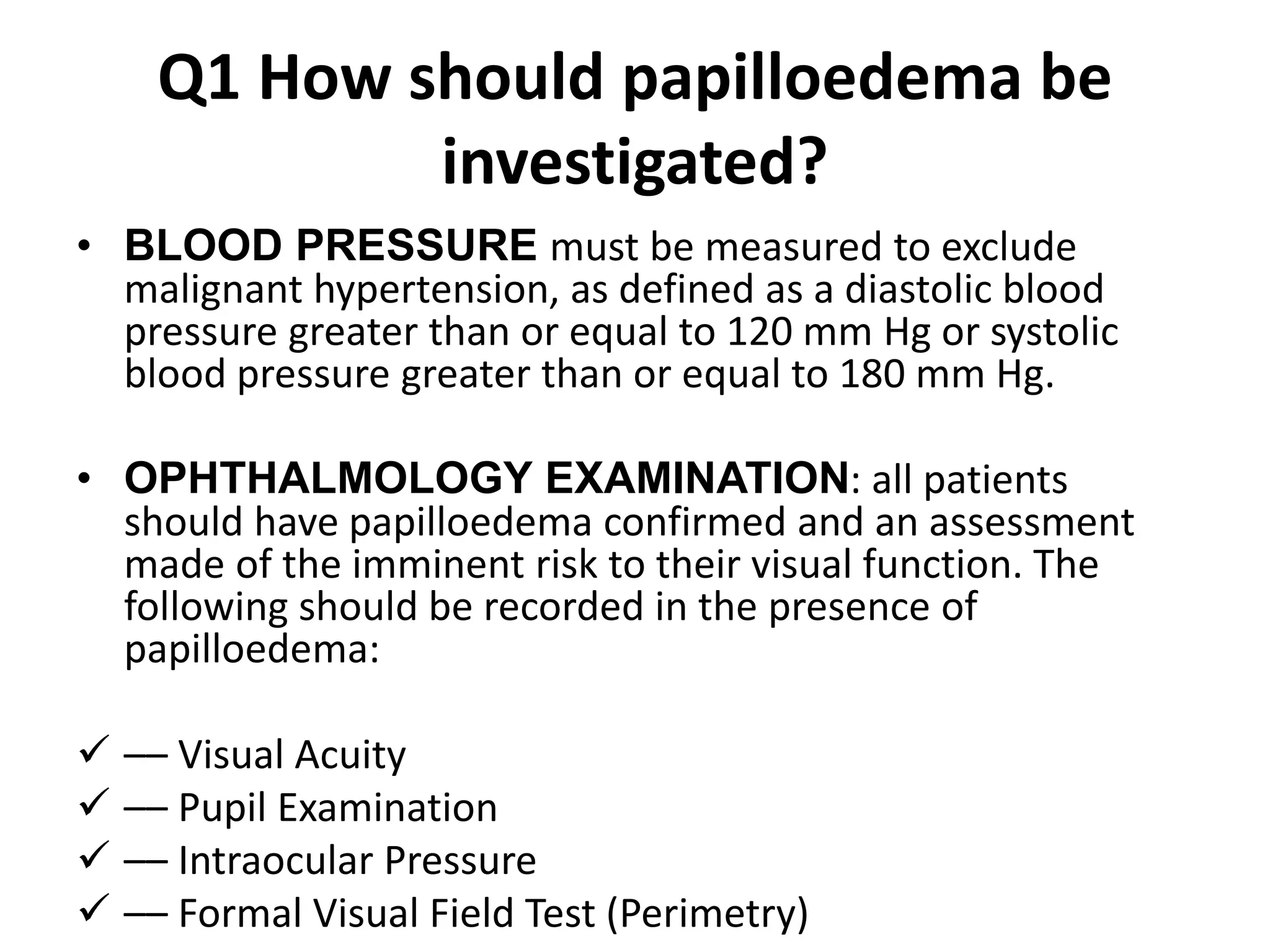
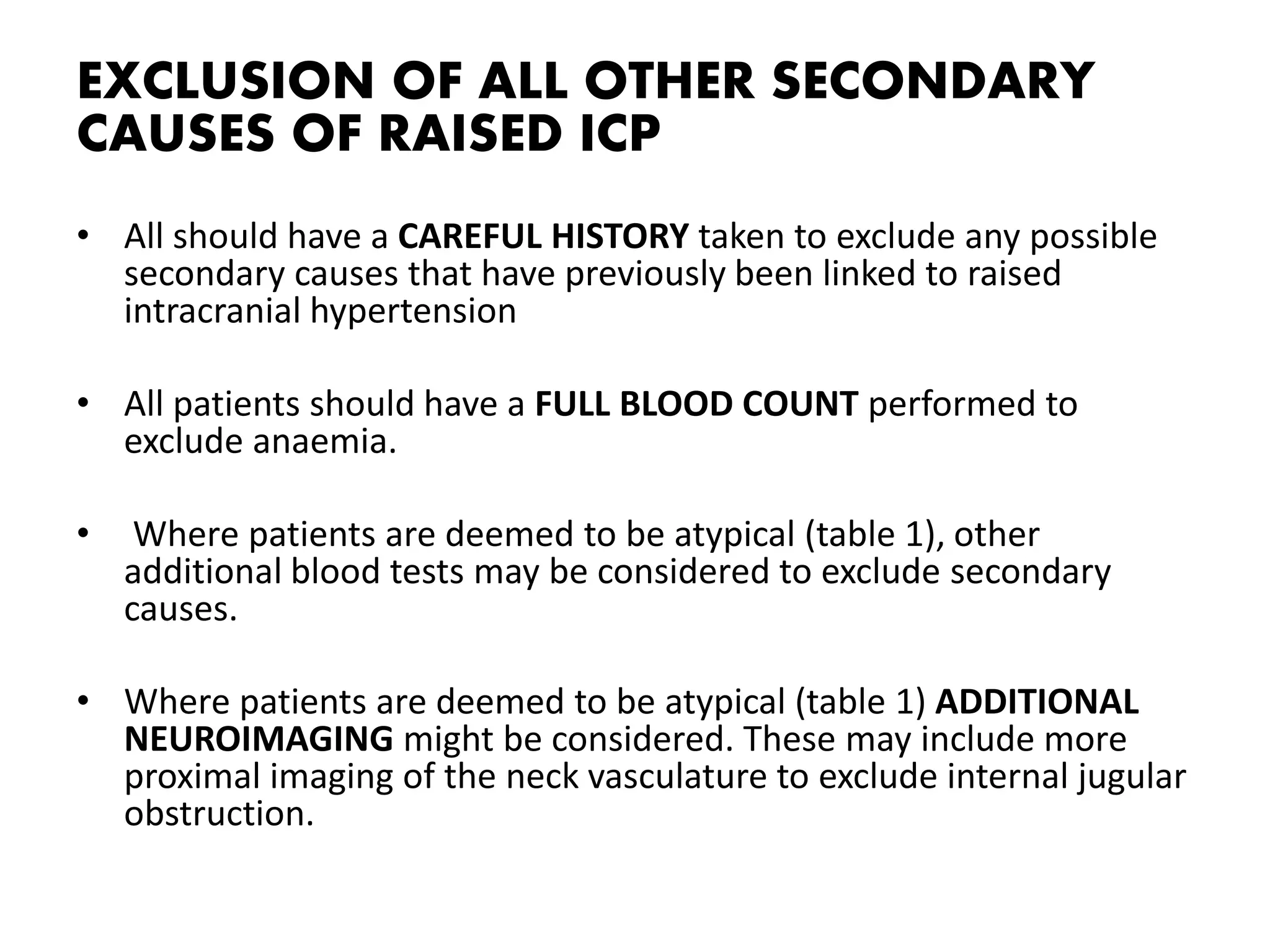
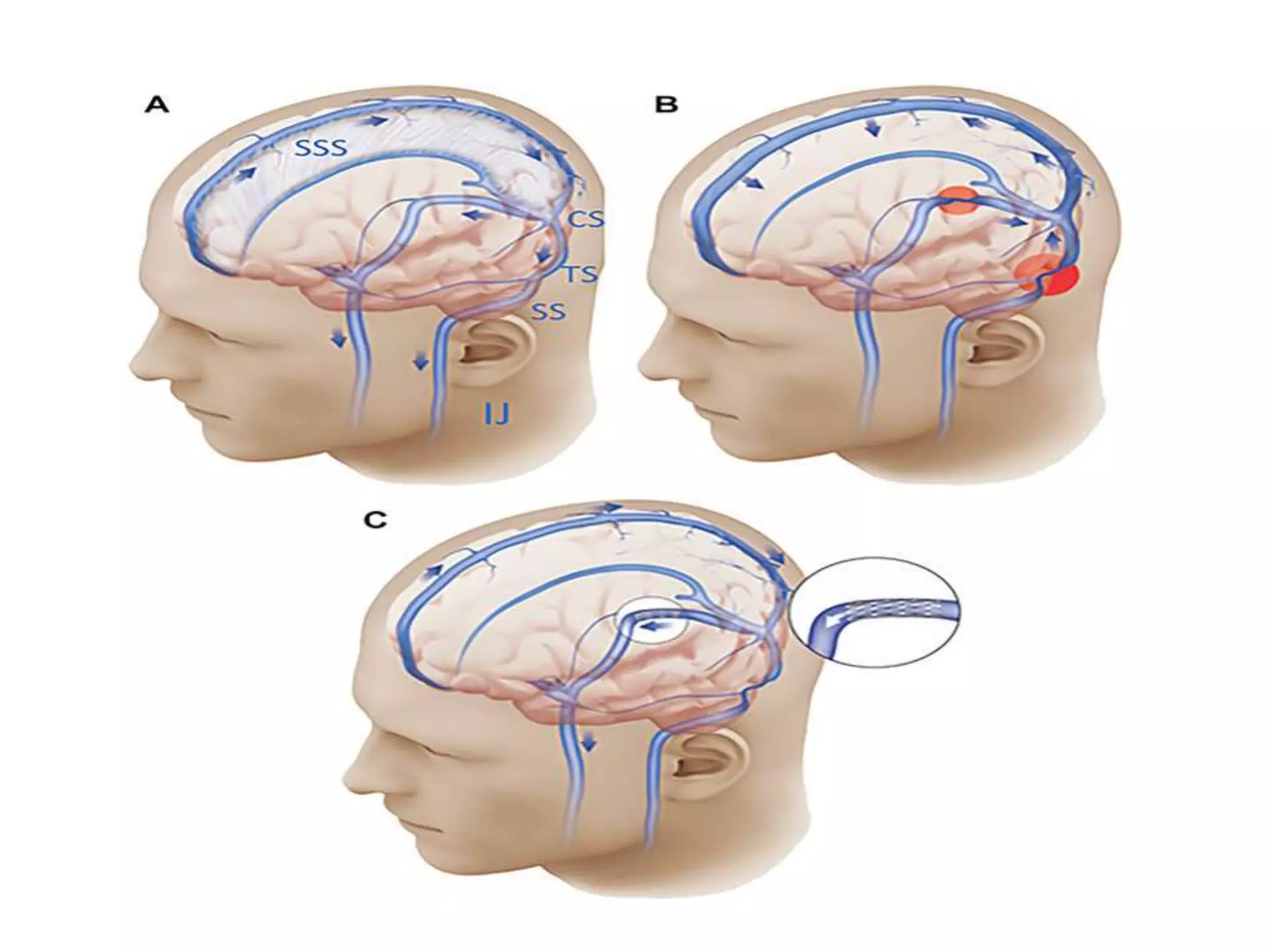
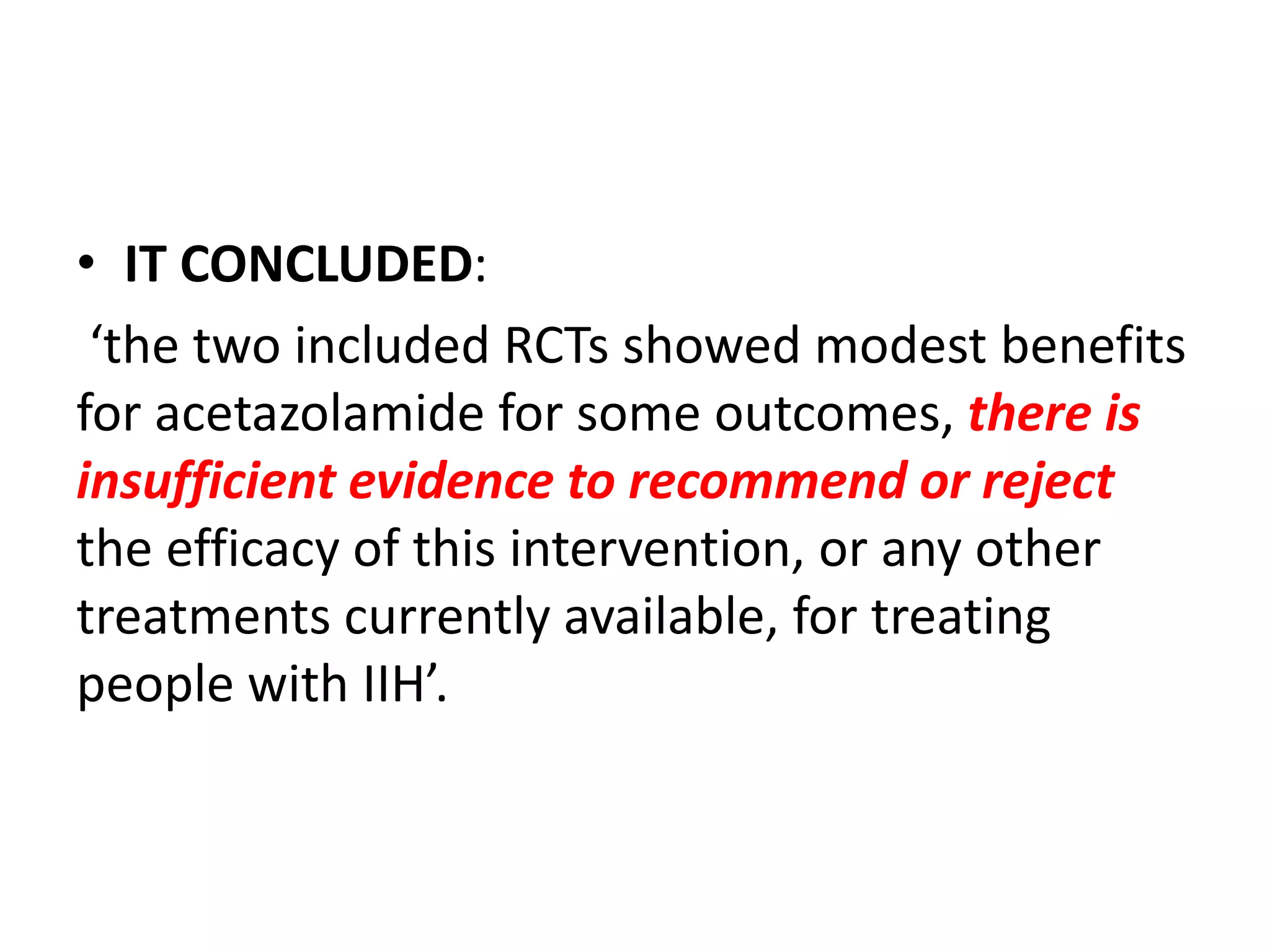
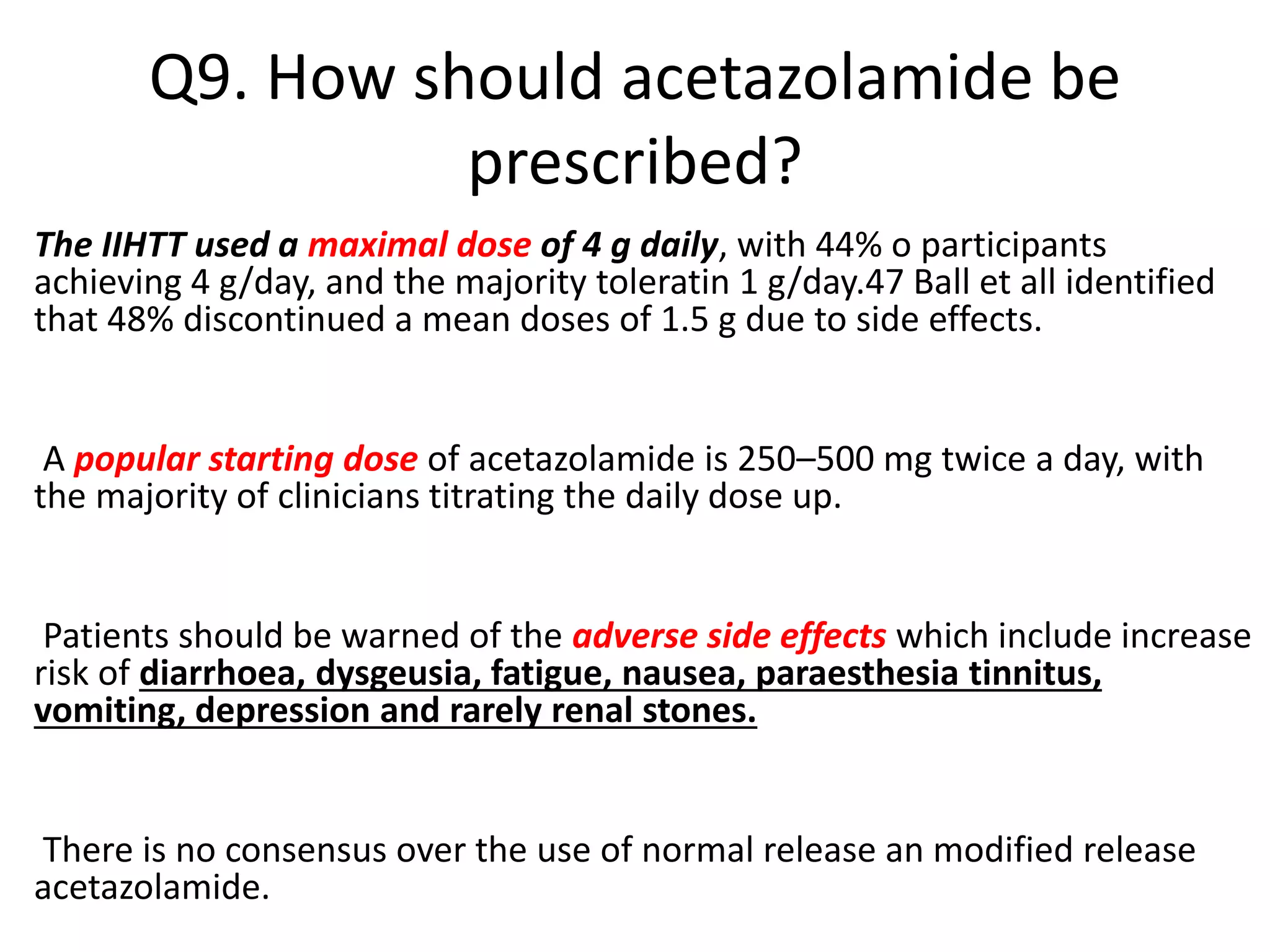
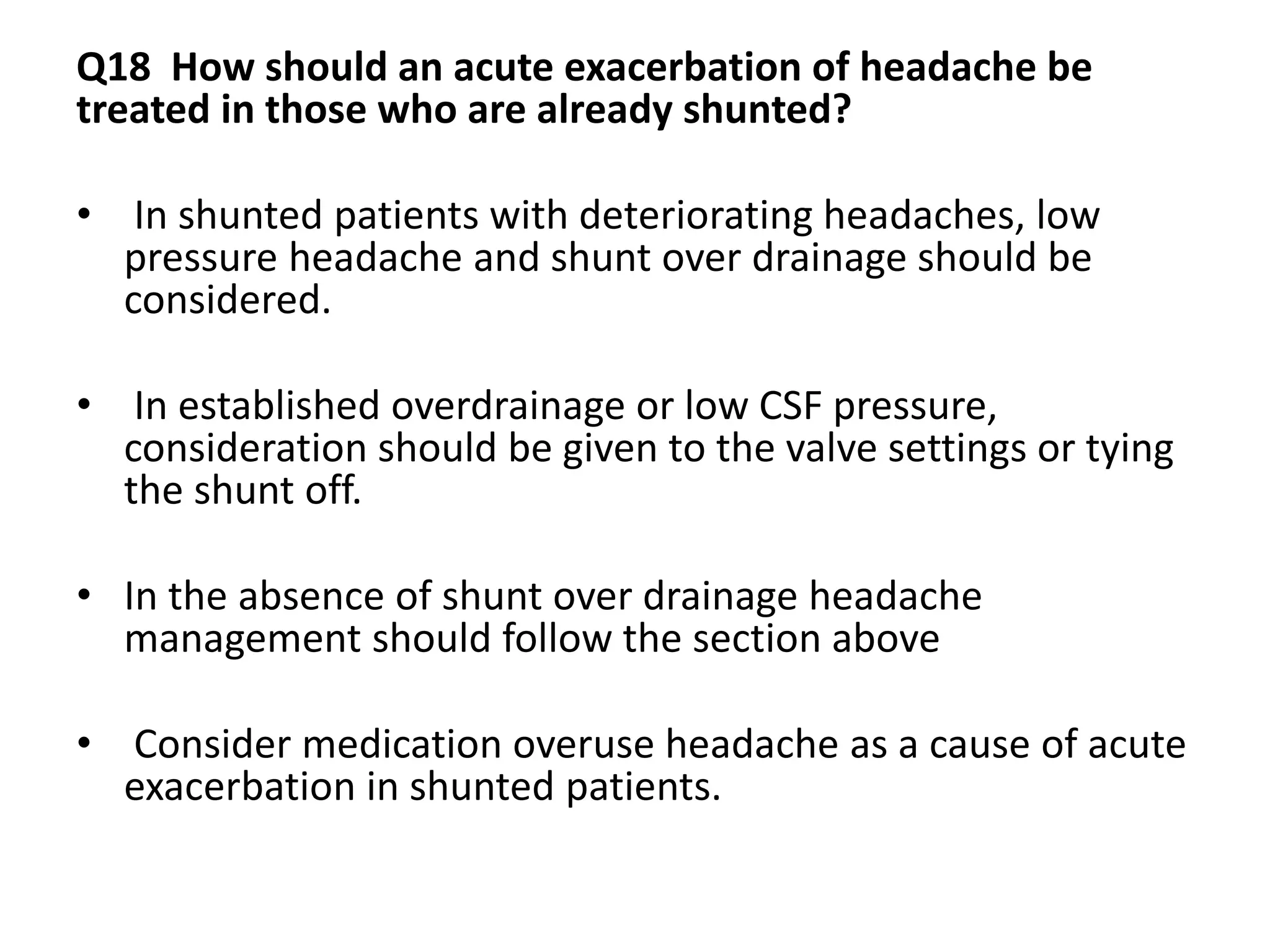
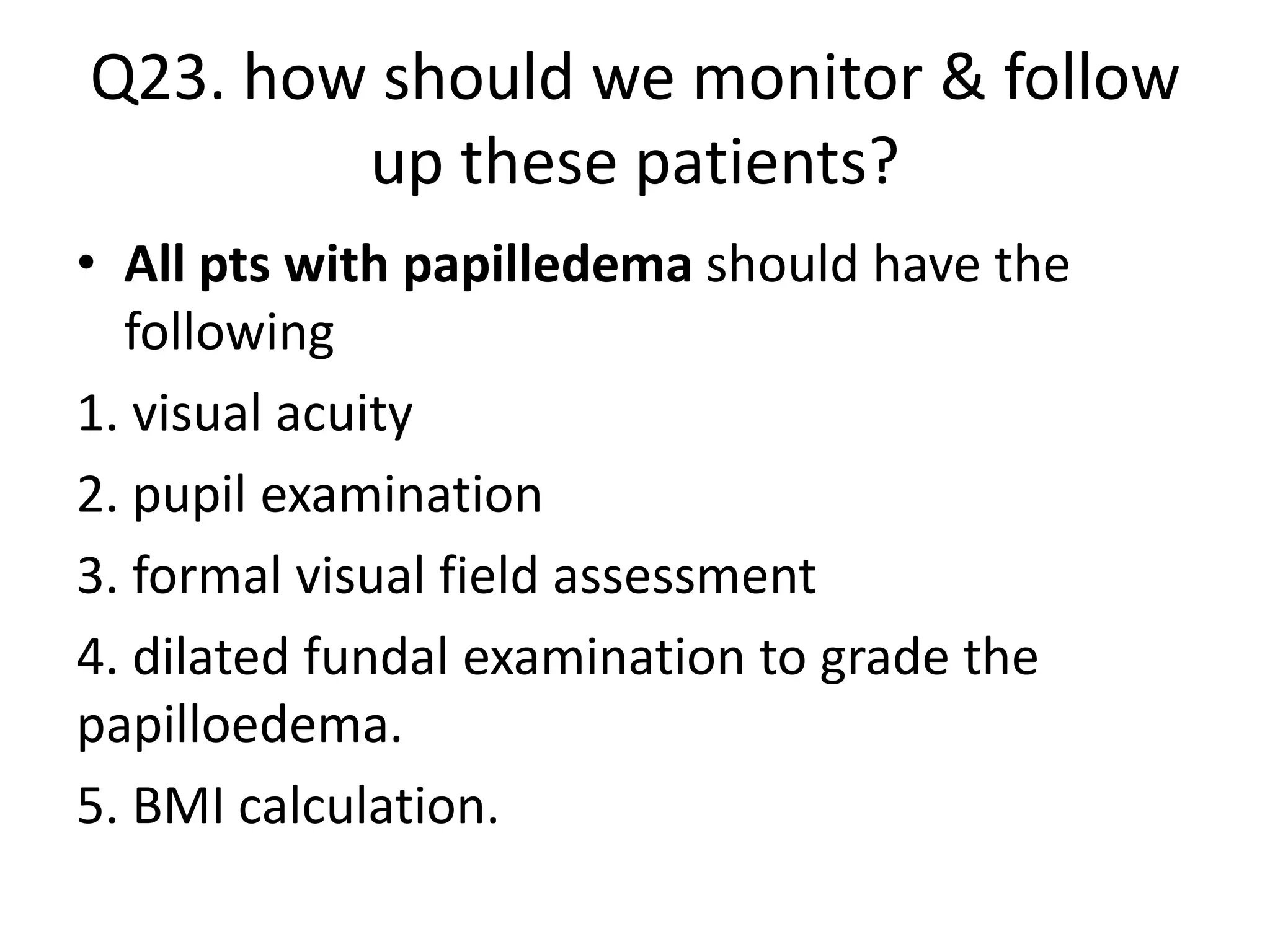
The document provides guidelines for investigating and managing papilloedema and idiopathic intracranial hypertension (IIH). It recommends measuring blood pressure and performing ophthalmological and neurological exams. Neuroimaging should include MRI or CT with venography to rule out other causes. Lumbar puncture criteria include opening pressure >25 cm CSF. Weight loss through lifestyle changes is the primary treatment, while acetazolamide may help headaches. Surgery like CSF shunting is considered for acute vision loss.