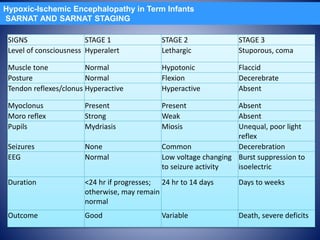
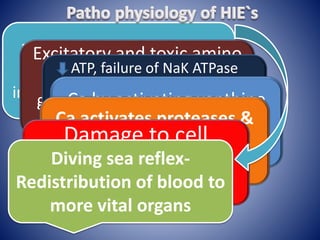
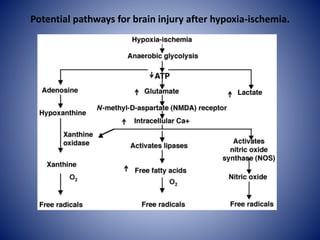
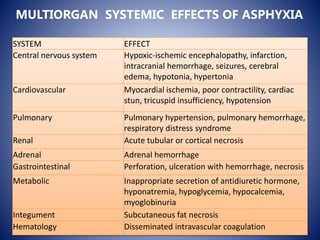
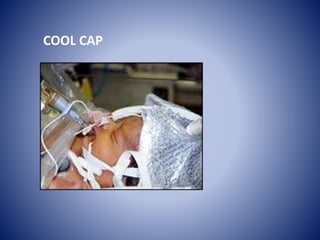
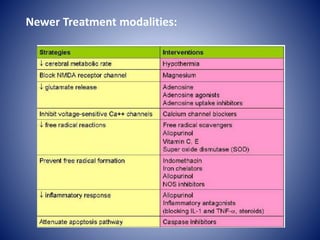
This document discusses hypoxic ischemic encephalopathy (HIE), which occurs when a full-term infant experiences significant intrapartum asphyxia. Causes of HIE include maternal cardiac arrest, placental abruption, and fetal hemorrhage. Without treatment, 15-20% of infants with HIE die in the neonatal period, and 25-30% are left with permanent disabilities. Diagnosis is based on metabolic acidosis at birth, early onset of encephalopathy symptoms, and multi-organ dysfunction. HIE is managed through therapeutic hypothermia within 6 hours of birth, which reduces brain injury by decreasing cerebral metabolism and cell death.