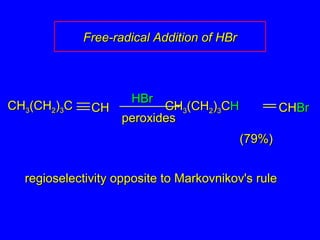
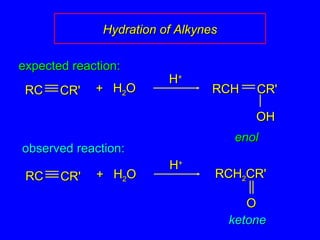
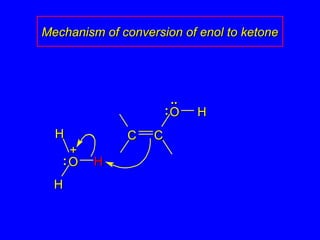
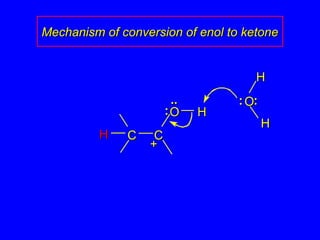
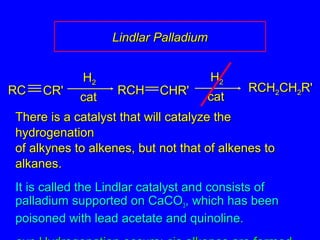
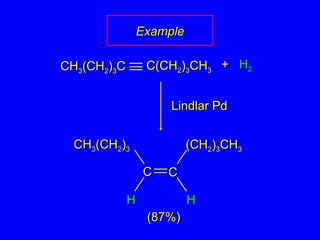
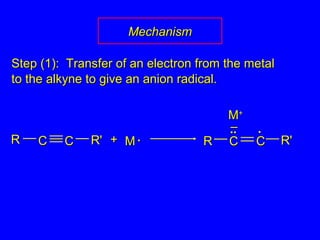
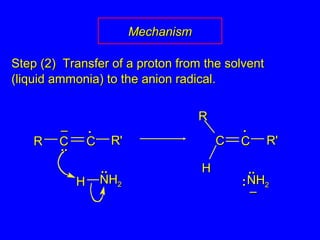
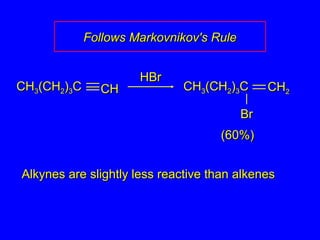
The document outlines various reactions of alkynes, including hydrogenation, metal-ammonia reduction, and addition of hydrogen halides, as well as the mechanisms involved in these reactions. It describes specific catalysts, such as Lindlar catalyst, and the regioselectivity demonstrated in reactions like hydration and addition of halogens. Key examples and mechanisms highlight the conversion of alkynes to alkenes and ketones, emphasizing stability and equilibrium in these reactions.
















![CHCH
....
BrBrHH ::
....
RCRC
....
BrBrHH ::
....
Observed rate law: rate =Observed rate law: rate = kk[alkyne][HX][alkyne][HX]22
Termolecular Rate-Determining StepTermolecular Rate-Determining Step](https://image.slidesharecdn.com/hydrogenation4-170419130242/85/Hydrogenation-4-22-320.jpg)