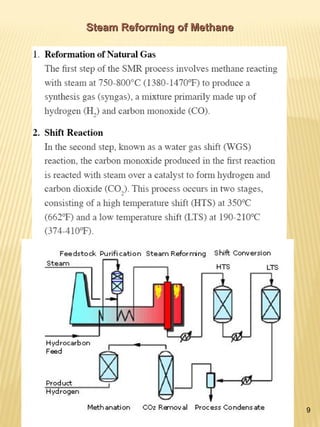
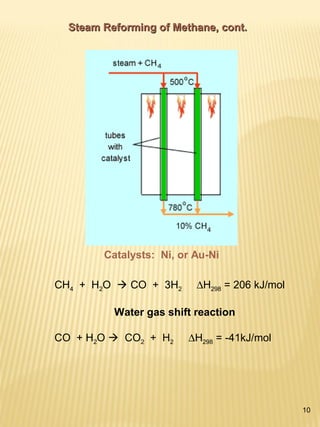
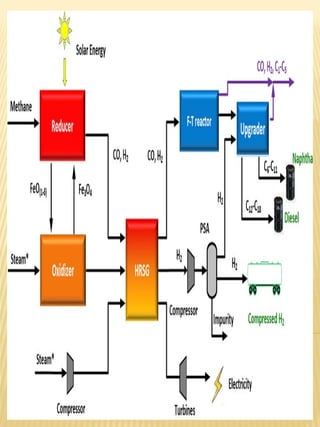
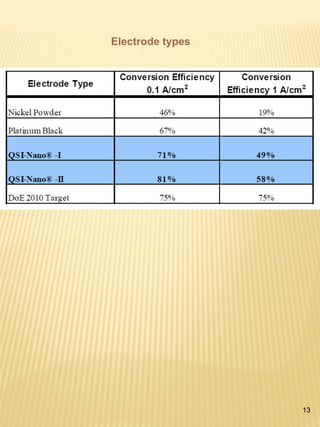
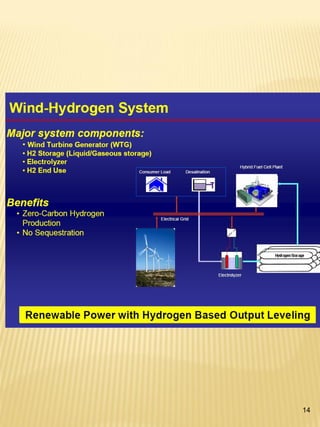
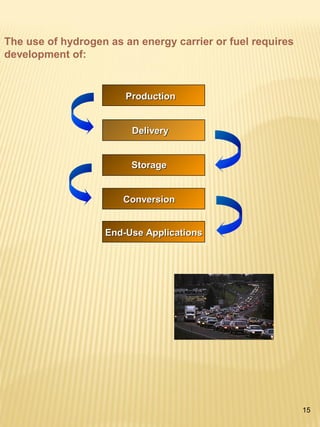
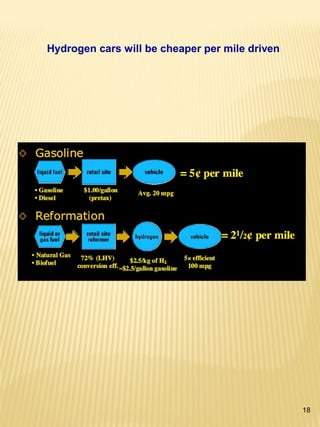
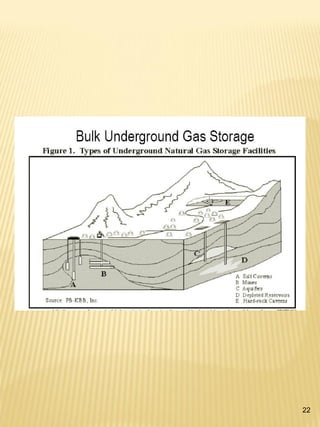
Hydrogen has the potential to be a sustainable energy carrier. It is abundant in the universe and can be produced from water through electrolysis, which splits water into hydrogen and oxygen using electricity. Currently, most hydrogen is produced through the energy-intensive process of steam reforming natural gas. Hydrogen is highly versatile as an energy carrier and can be used in various applications like transportation, where it can power fuel cell vehicles or be used in internal combustion engines. However, for hydrogen to be widely adopted challenges remain in its production, delivery, storage and conversion to useful energy in applications. Overall, hydrogen has promising characteristics as a renewable fuel but more development is needed in the technologies around its infrastructure and end uses.