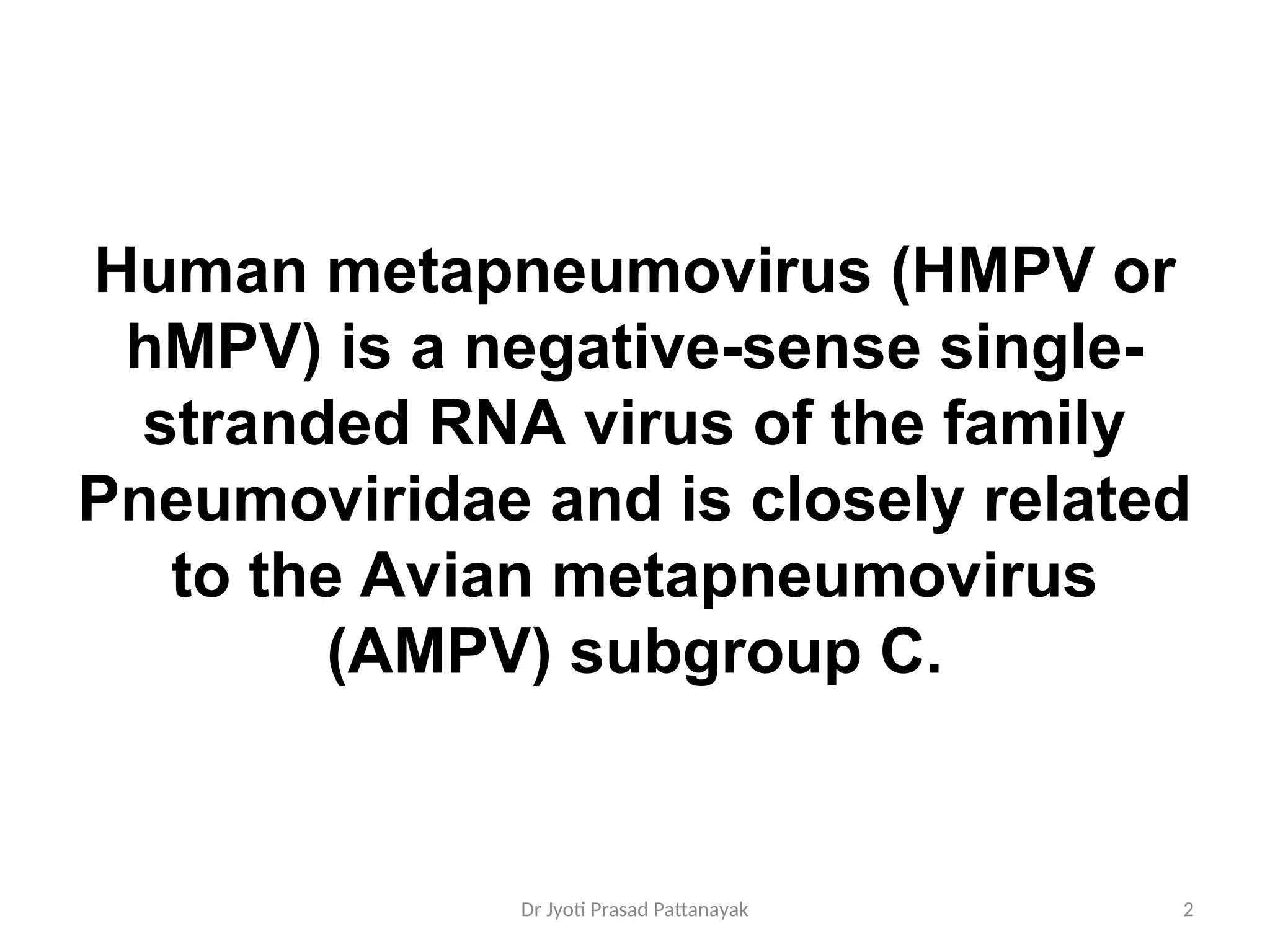
Human metapneumovirus (HMPV) is a negative-sense RNA virus that primarily causes respiratory illnesses, particularly in children and older adults. It was first isolated in 2001 and has been linked to significant respiratory infections, with outbreaks observed and a seasonal pattern of prevalence. Diagnosis mainly relies on RT-PCR technology, and no specific treatment is currently available, though ribavirin shows promise in models.