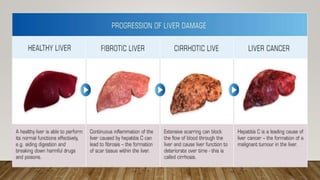
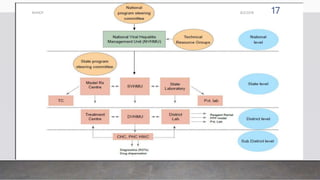
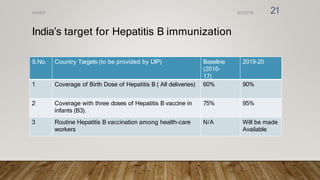
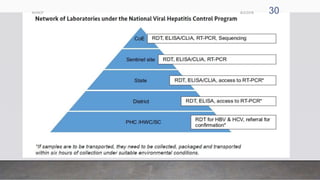
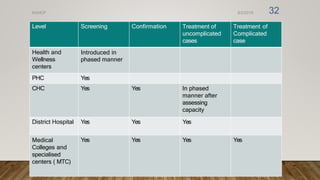
The document outlines the National Viral Hepatitis Control Program (NVHCP) in India. The objectives of the NVHCP are to [1] enhance awareness of hepatitis, [2] provide early diagnosis and management of viral hepatitis at all healthcare levels, and [3] strengthen infrastructure and human resources for comprehensive hepatitis services. The program will implement strategies like immunization, harm reduction, and infection control to prevent hepatitis and establish treatment centers to diagnose and treat hepatitis cases.