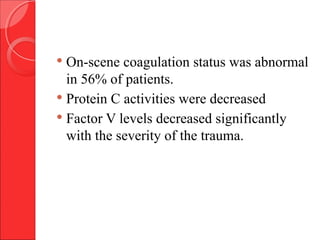
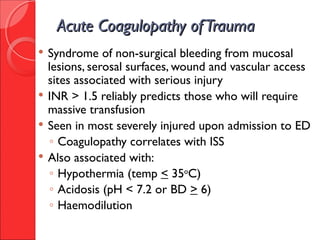
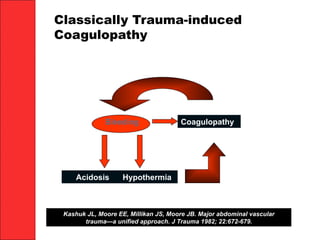
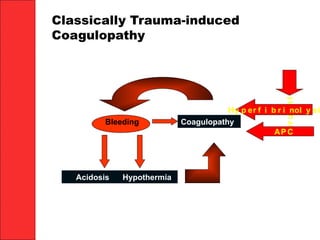
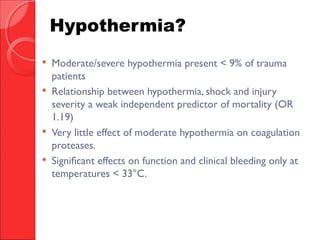
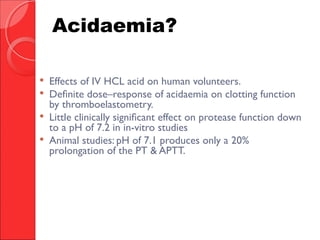
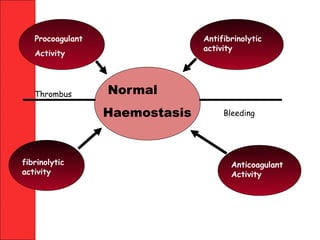
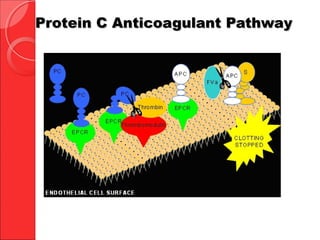
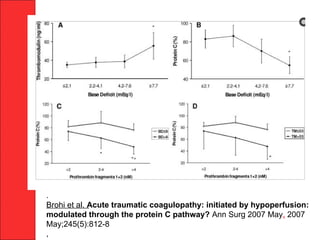
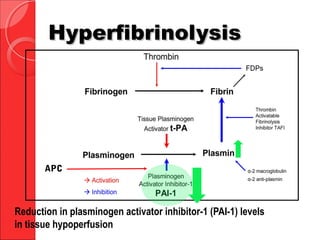
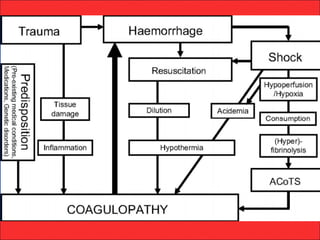
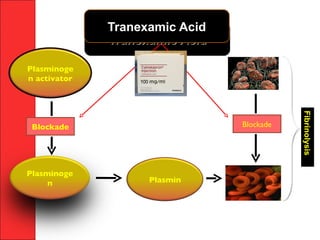
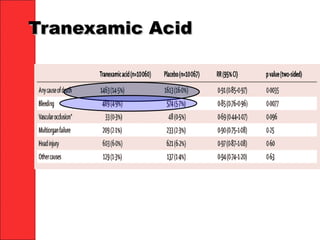
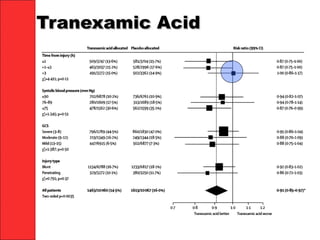
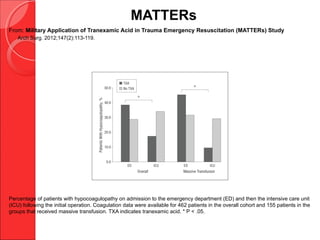
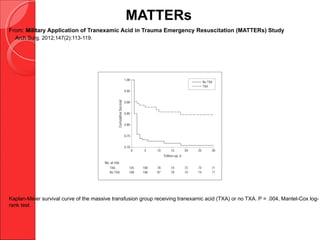
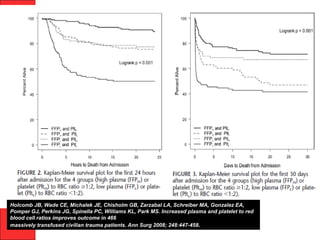
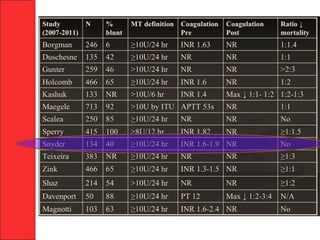
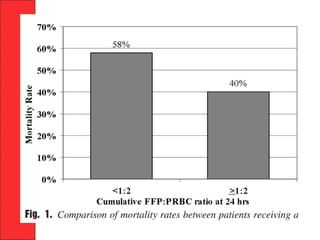
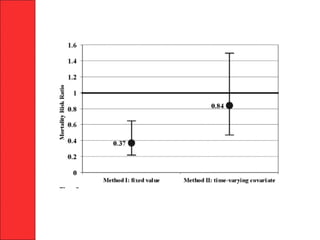
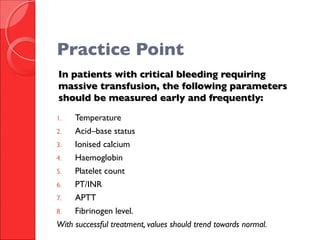
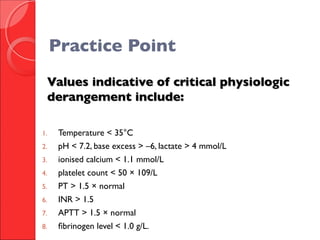
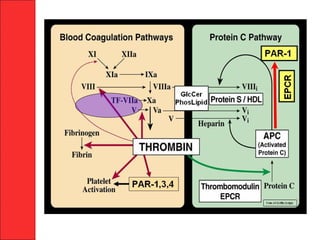
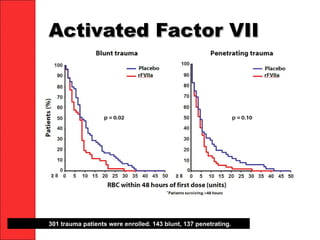
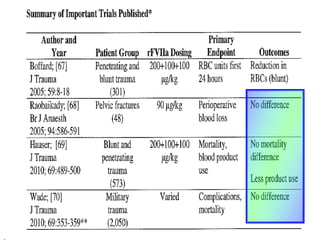
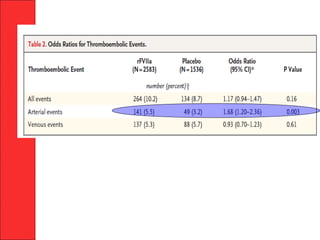
The document discusses the significant impact of traumatic coagulopathy in trauma patients, emphasizing its prevalence, especially in cases of severe injuries. It presents findings from various studies on coagulation profiles at accident scenes and during hospital admissions, identifying predictors of mortality and the importance of timely intervention. Recommendations for managing massive transfusions and the use of tranexamic acid to reduce mortality in bleeding patients are also highlighted.