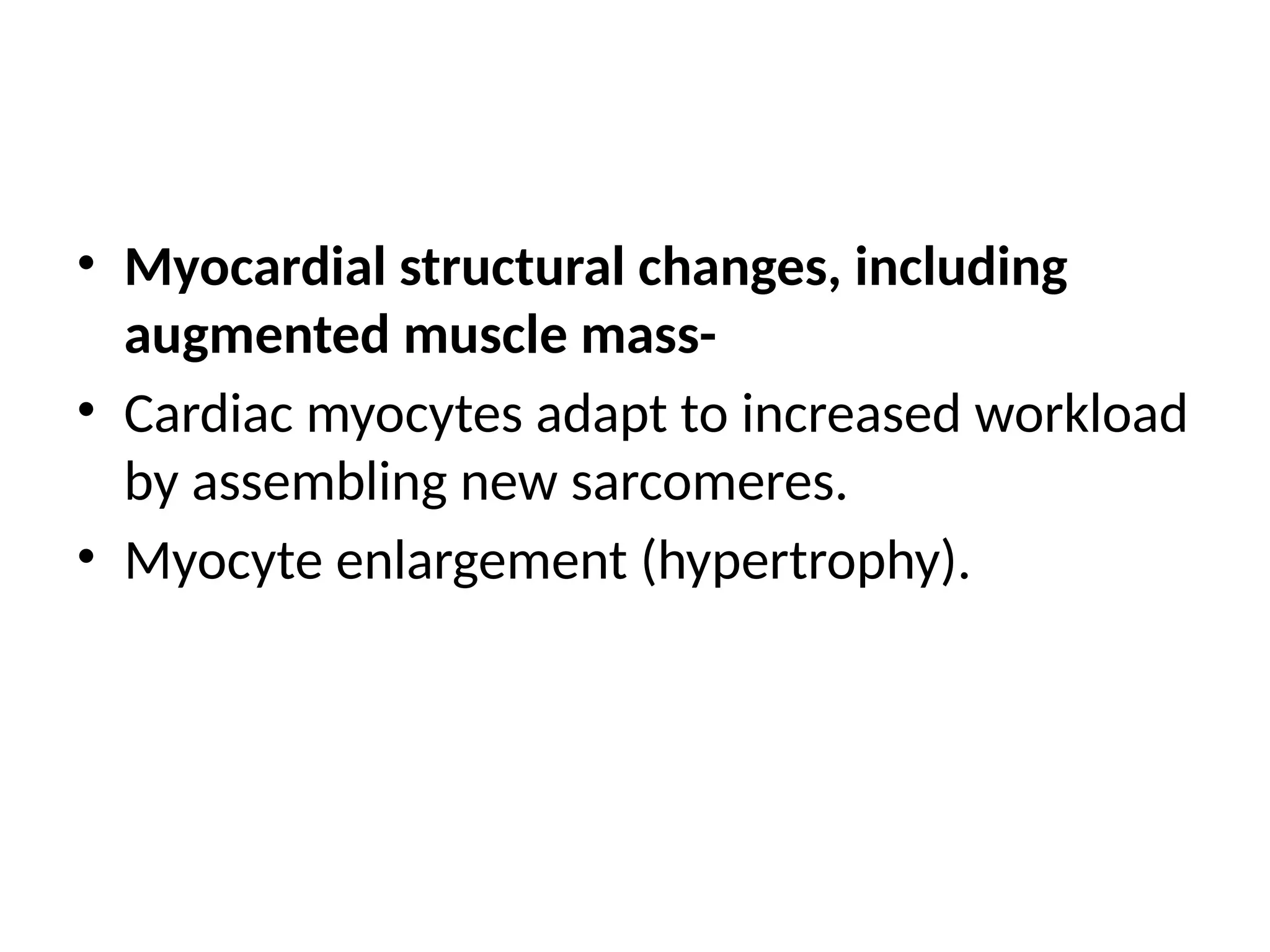
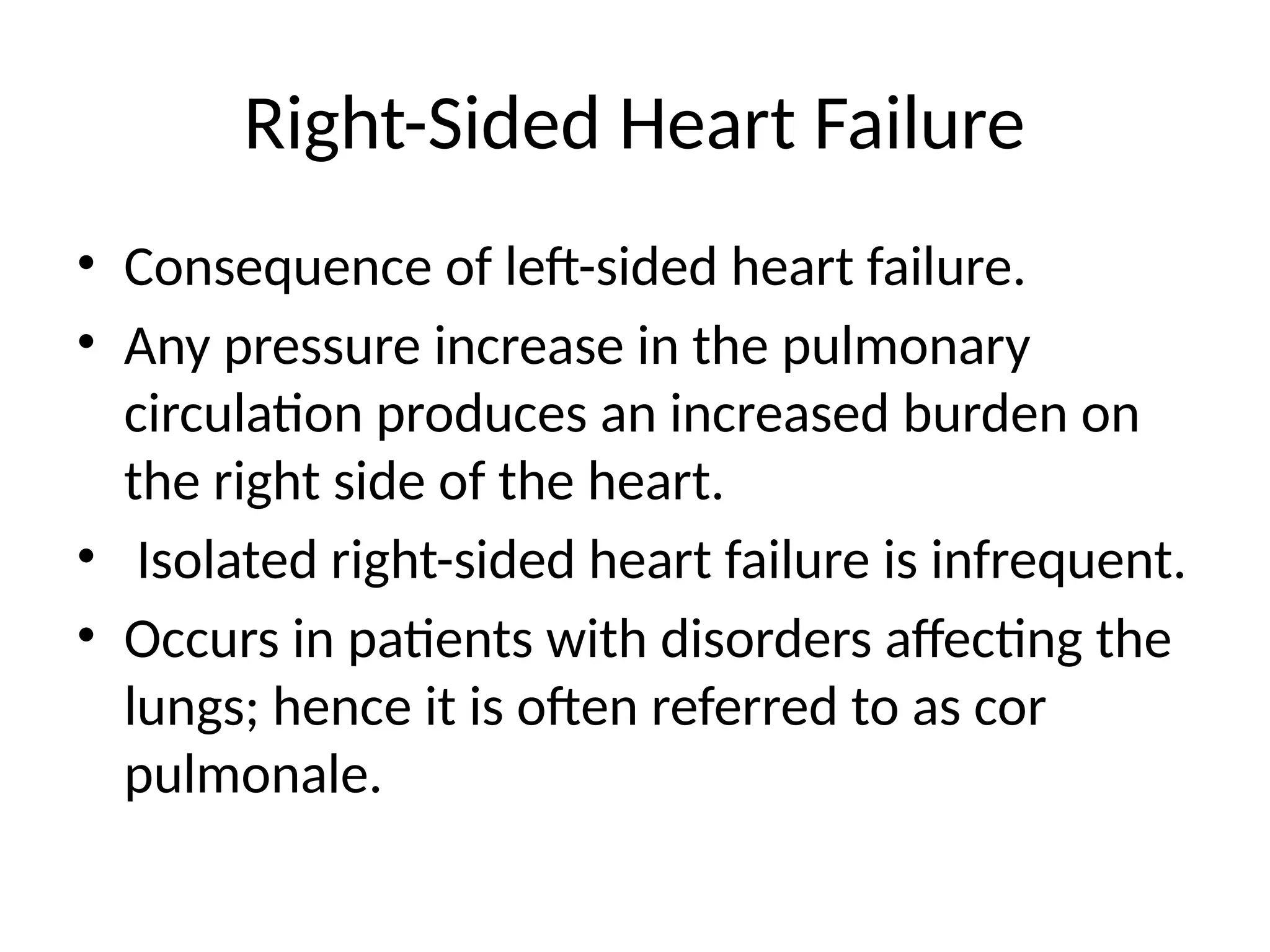
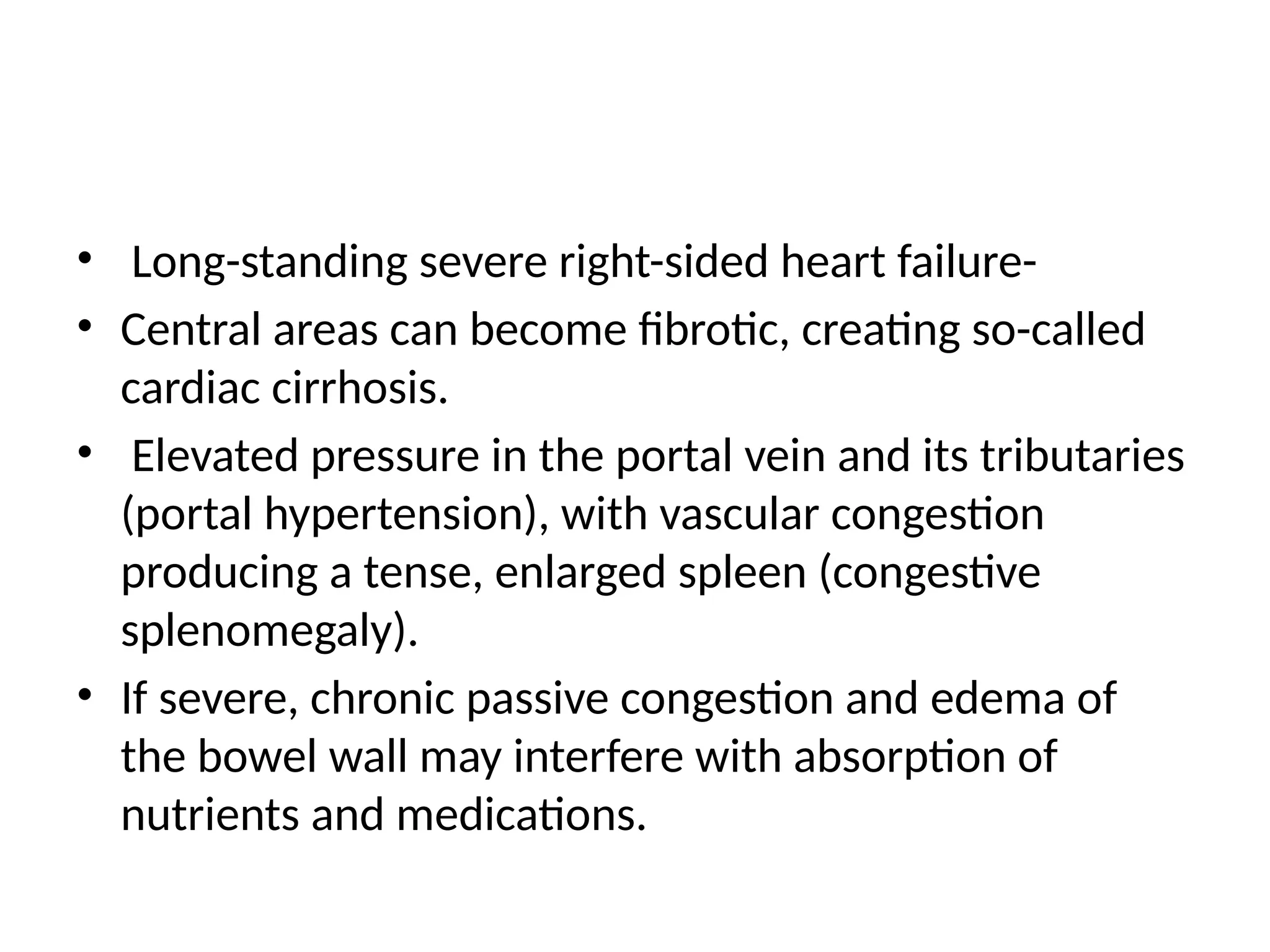
Heart failure, particularly congestive heart failure (CHF), is a progressive condition resulting from systolic or diastolic dysfunction due to various causes, including ischemic heart disease and hypertension. It can lead to inadequate cardiac output and increased venous congestion, affecting the left, right, or both sides of the heart, often resulting in symptoms such as dyspnea and cardiomegaly. Treatment focuses on correcting underlying causes, managing volume overload and improving myocardial contractility through pharmacologic agents and cardiac therapies.