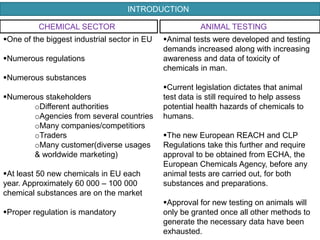
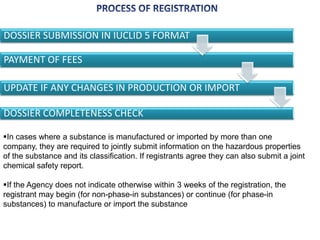
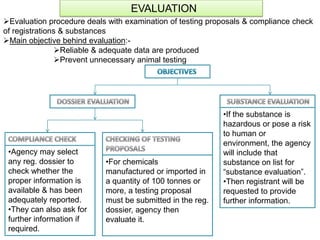
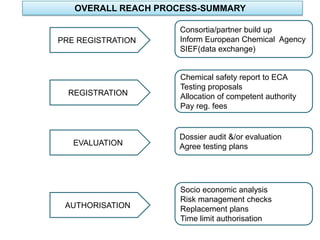
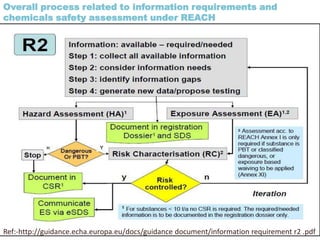
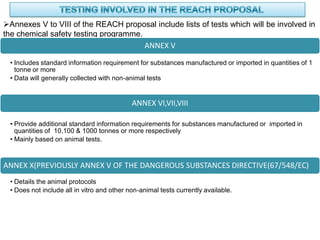
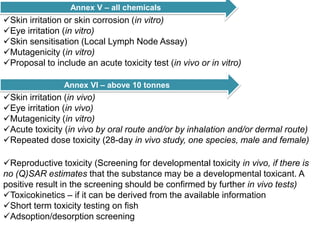
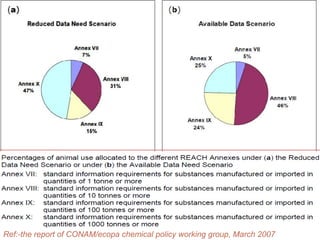
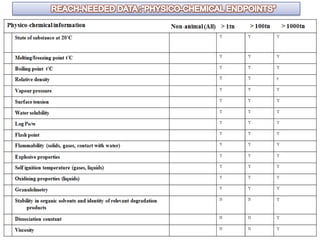
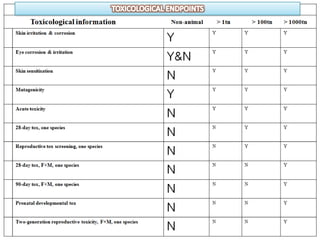
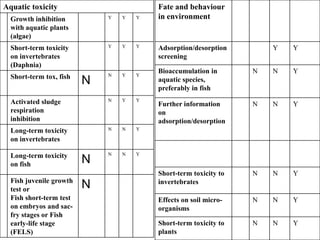
The document outlines the EU regulation REACH, which governs the registration, evaluation, authorization, and restriction of chemicals to protect human health and the environment, while also fostering innovation in the chemical industry. It specifies the data requirements for testing the safety of chemical substances, including both animal and alternative testing methods, and details the process for evaluating chemical hazards and granting authorization for substances of very high concern. The document emphasizes the necessity of compliance with these regulations to ensure the safe use of chemicals and encourages the use of alternative assessment approaches to reduce animal testing.