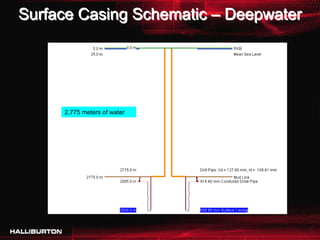
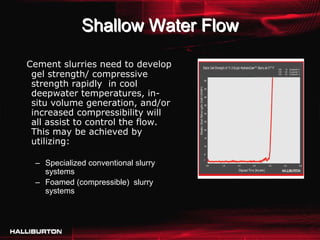
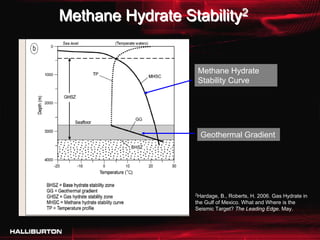
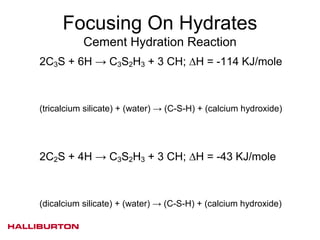
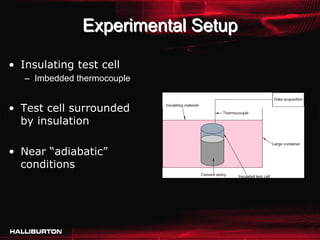
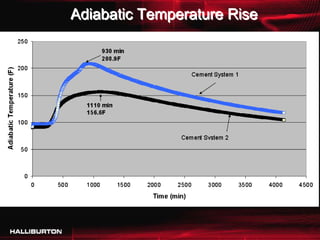
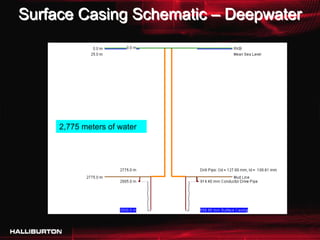
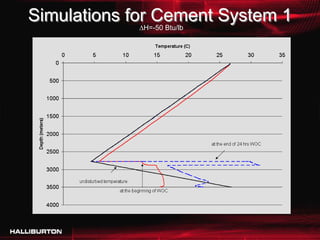
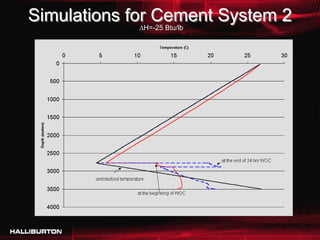
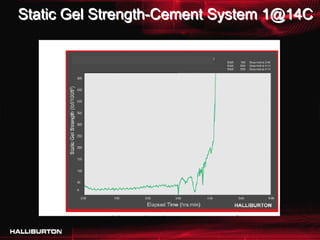
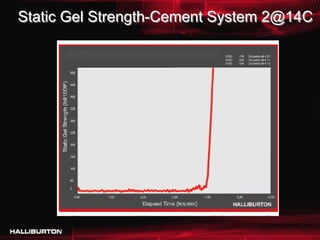
This document discusses challenges with cementing in deepwater environments, including shallow water flows and gas hydrate destabilization due to cement heat of hydration. It presents a solution of optimizing cement slurry design to reduce heat of hydration through lowering the calcium silicate reaction enthalpies. Experimental data shows that a cement system designed with a lower heat of hydration reduces temperature rise and develops strength rapidly enough to prevent issues. This optimized system was successfully implemented in the field to help prevent gas hydrate destabilization during cementing.