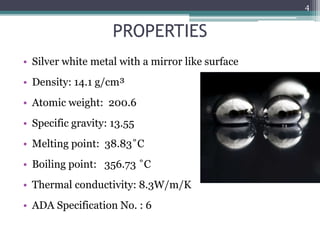
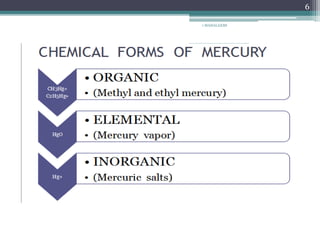
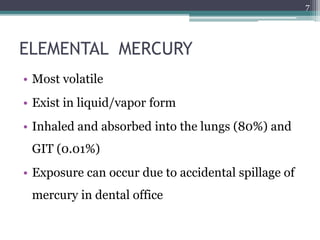
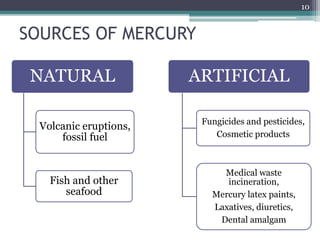
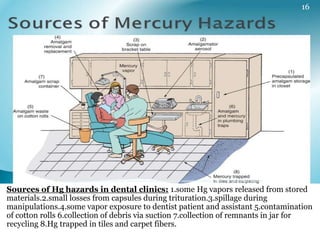
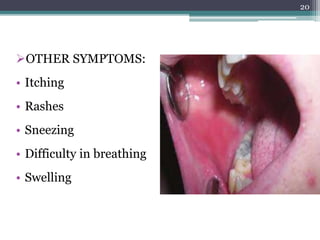
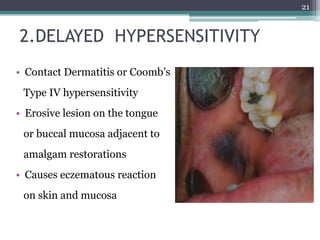
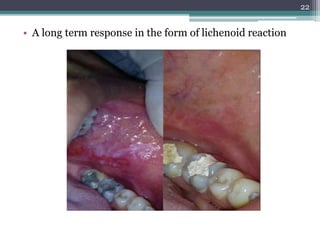
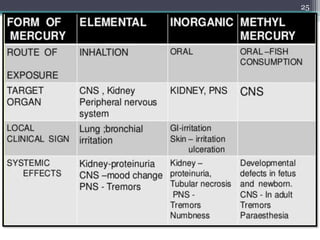
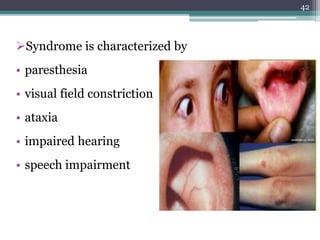
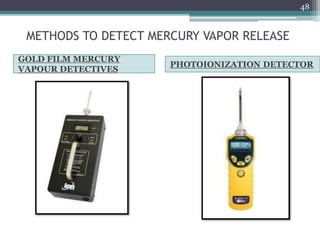
This document provides information about mercury, its various forms, sources of exposure, toxicity, and hygiene practices related to dental use. It begins with properties of mercury and discusses its common uses in dental amalgam. Sources of mercury exposure include elemental, inorganic, and organic forms. Health effects of mercury poisoning can be allergic, acute, or chronic depending on dosage and length of exposure. The document outlines methods to detect mercury vapor, treatment for toxicity, and hygiene recommendations to minimize exposure in dental settings.