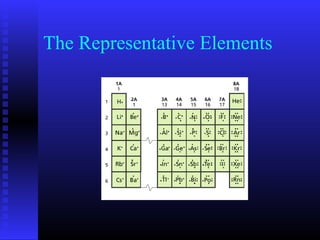
The document summarizes the organization and properties of the modern periodic table. It is organized into blocks that include metals, nonmetals, metalloids, representative elements, transition metals, and inner transition metals. Key points about the properties and common uses of elements in each block are provided, such as lithium used in batteries, calcium essential for bones, and the noble gases being colorless and nonreactive.