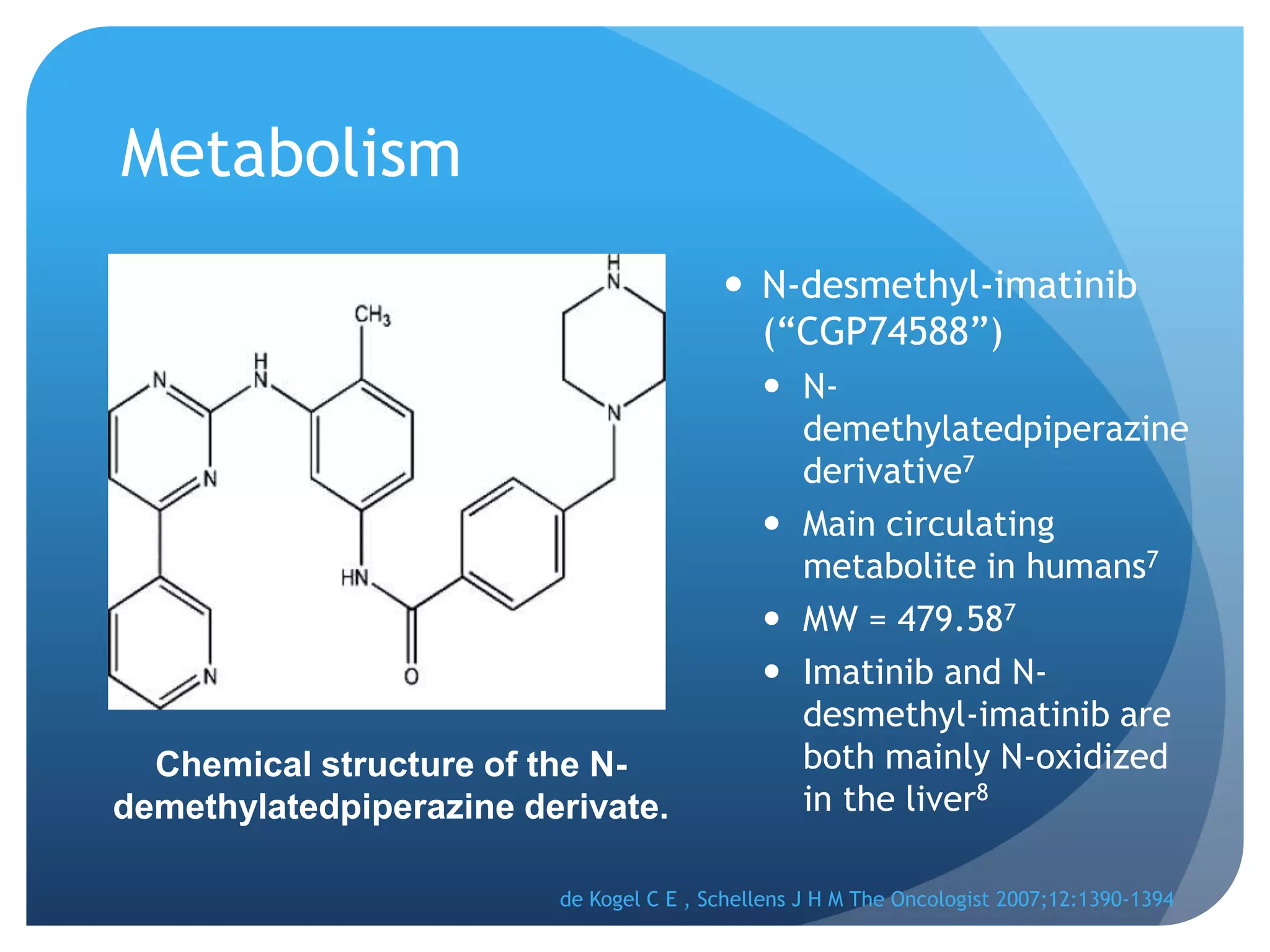
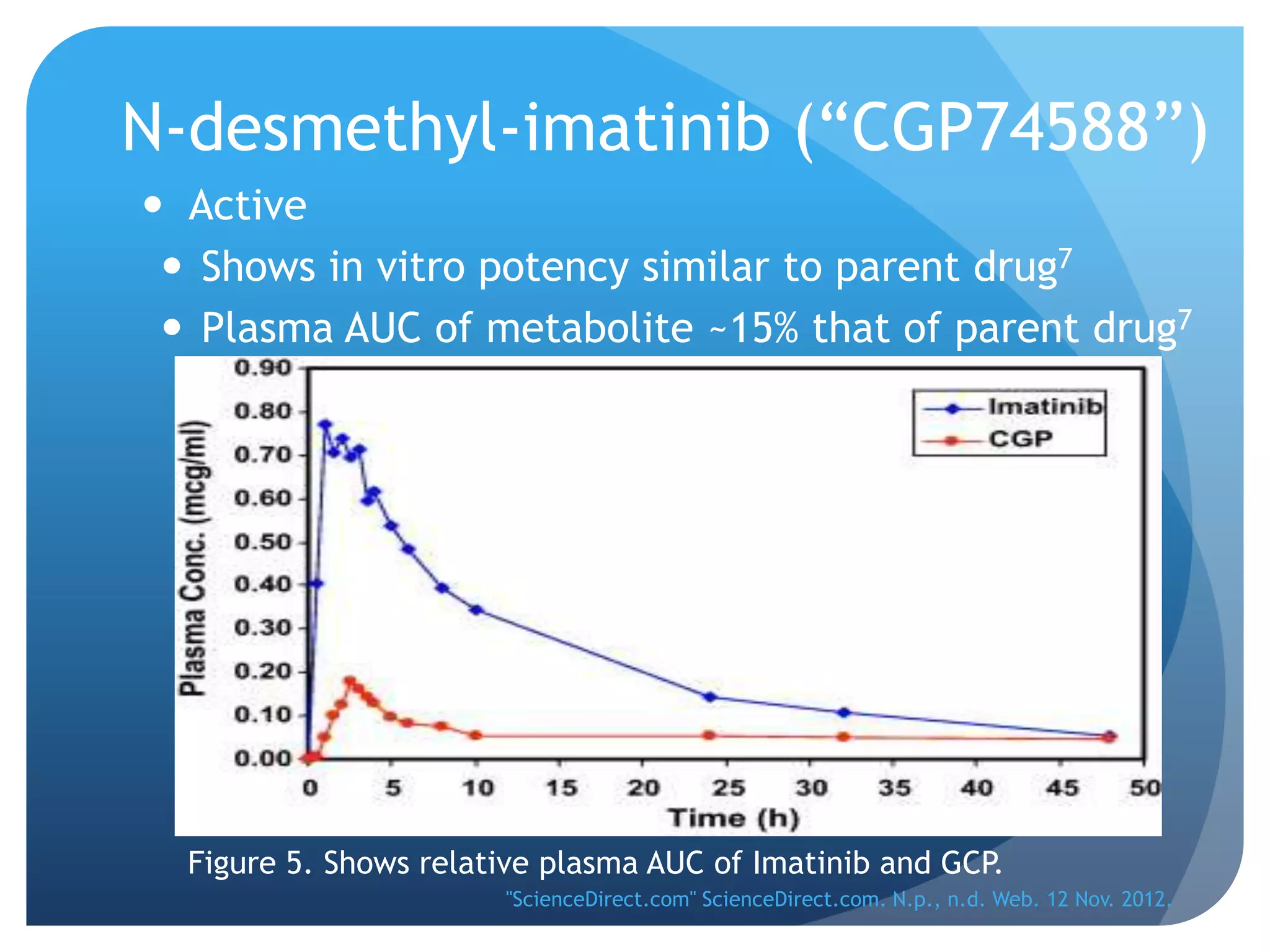
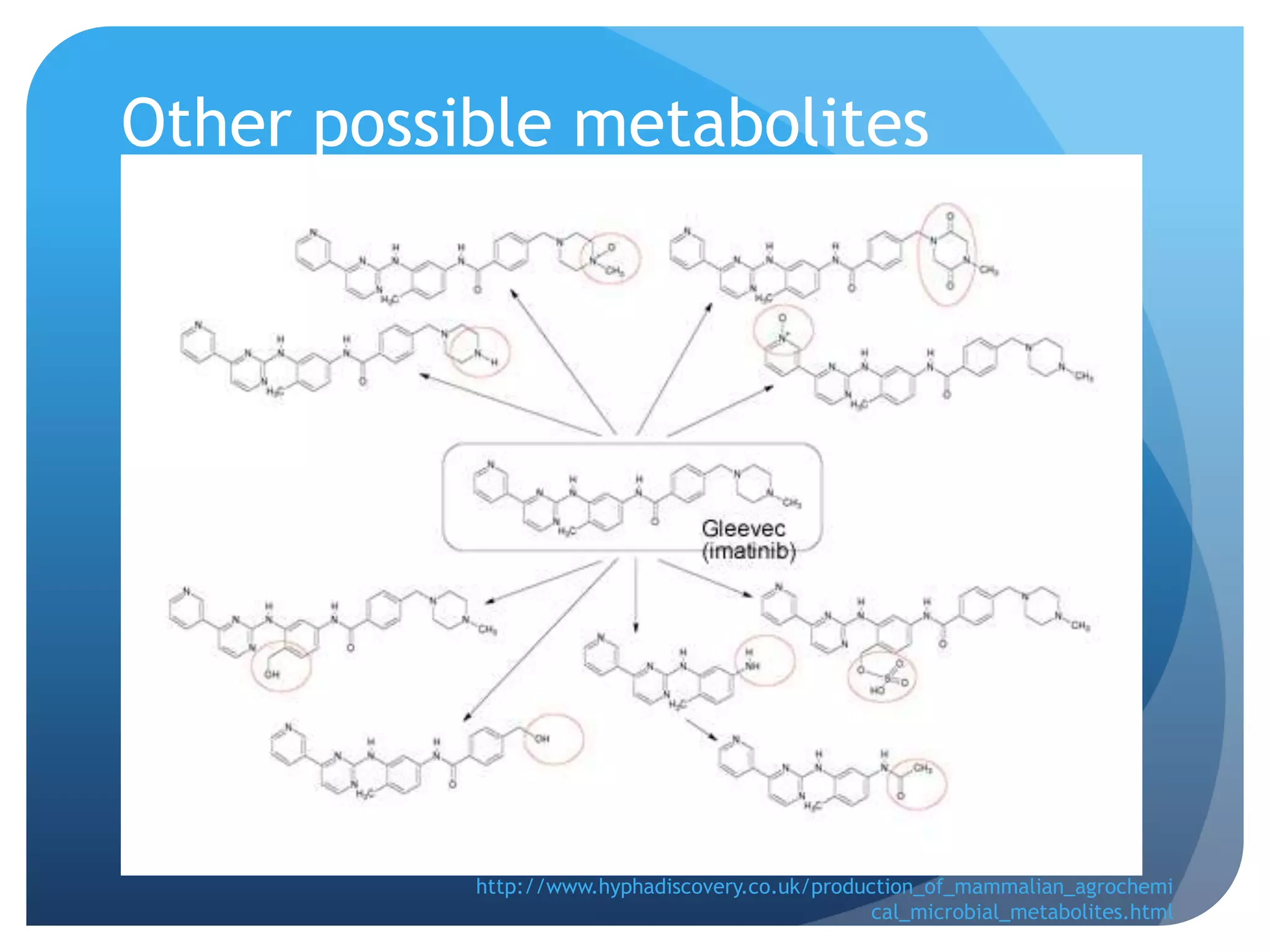
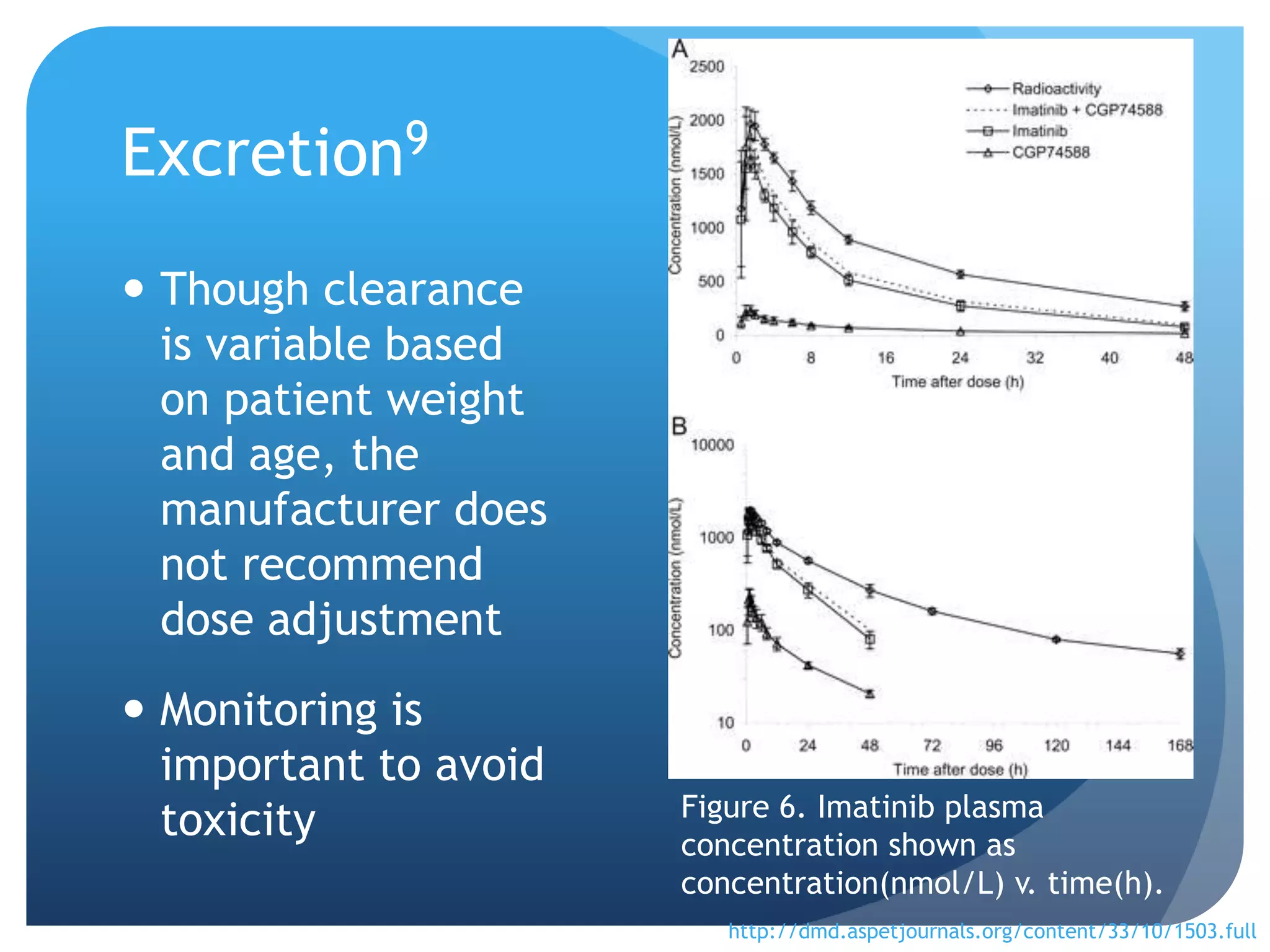
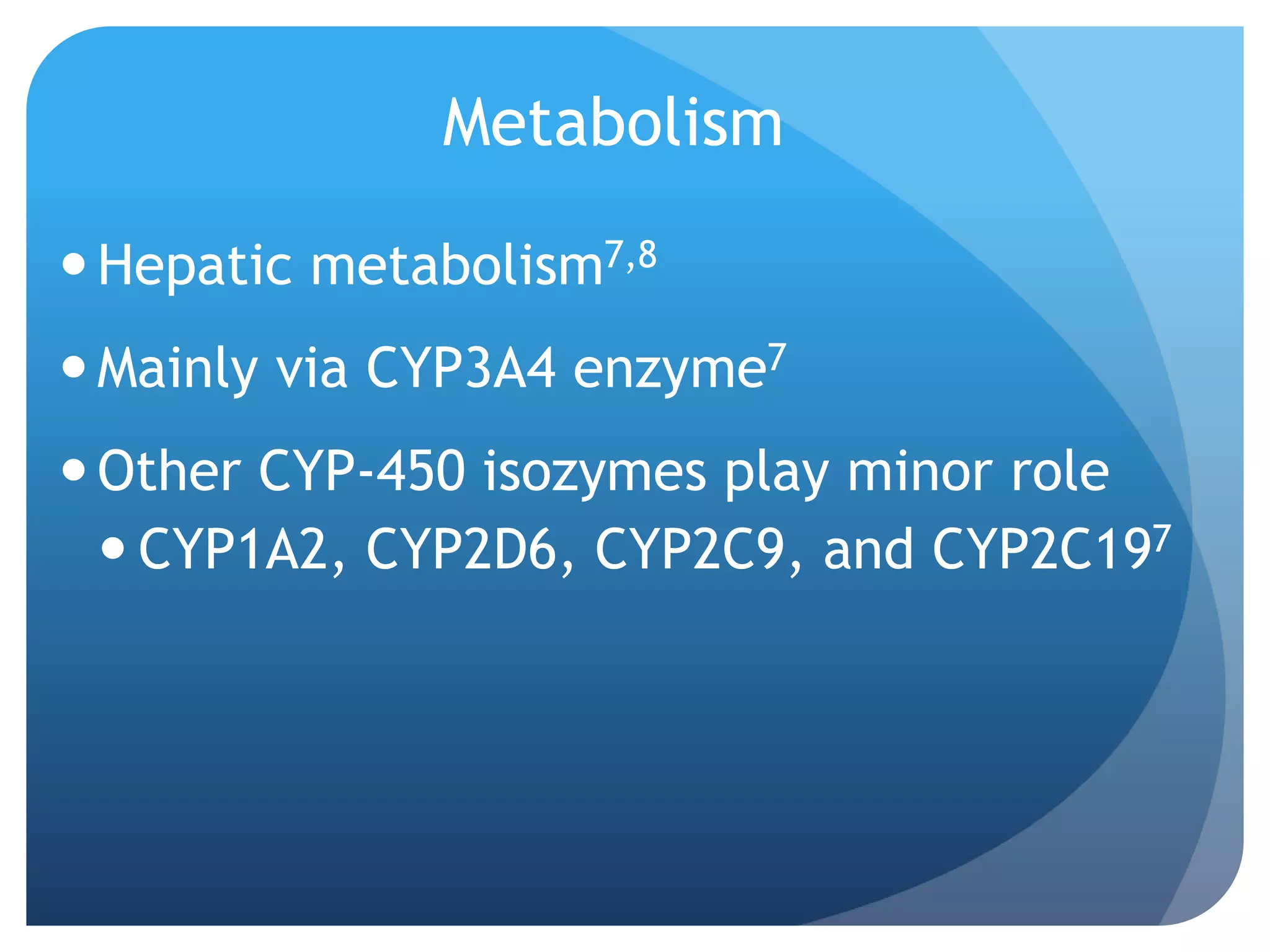
Imatinib (Gleevec) is a tyrosine kinase inhibitor developed to treat chronic myelogenous leukemia (CML). [1] It works by binding to the Bcr-Abl protein created by a chromosomal translocation, blocking its ability to phosphorylate proteins and activate cancer-causing pathways. [2-4] Imatinib is well-absorbed orally and highly protein bound. It undergoes hepatic metabolism primarily via CYP3A4 and is eliminated mostly in the feces. The main metabolite is an active N-demethylated derivative. Dose adjustments are generally not needed due to its variable clearance. [5-11]

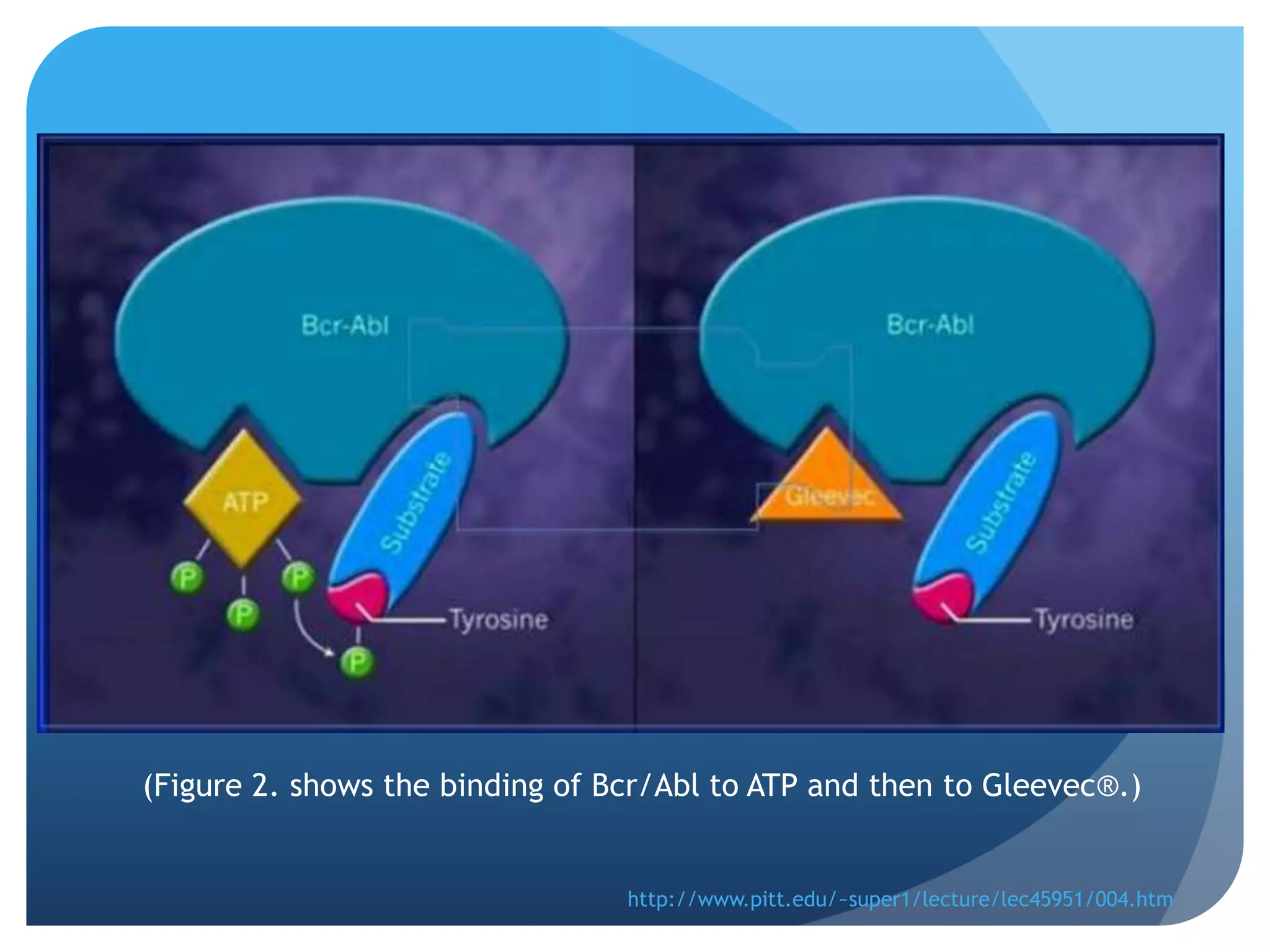




![Figure 4. Imatinib
location of
metabolism.
Picture: "Pathway: Imatinib
Pathway, Pharmacokinetics/Pharmacodynamics  [UNDER REVIEW]."
Imatinib Pathway, Pharmacokinetics/Pharmacodynamics [PharmGKB].
N.p., n.d. Web. 11 Nov. 2012.](https://image.slidesharecdn.com/gleevecgrouppresentation1-121202232829-phpapp01/75/Gleevec-group-presentation-1-14-2048.jpg)