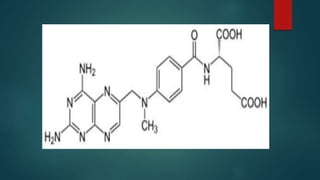
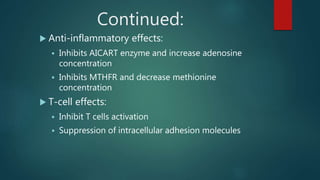
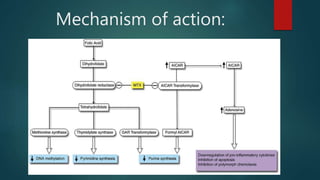
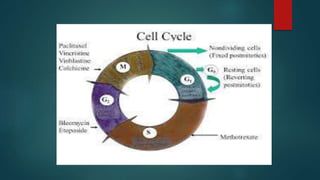
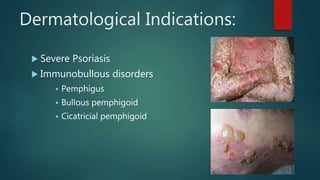
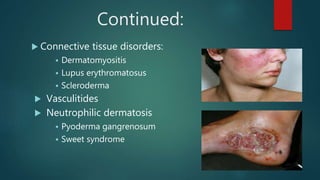
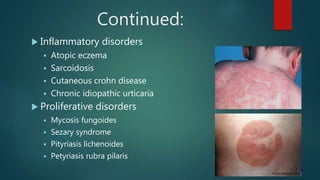
Methotrexate is a synthetic drug that interferes with cell growth and reduces inflammation. It is used as a chemotherapeutic, immunosuppressive, and anti-inflammatory agent to treat several dermatological conditions like psoriasis, immunobullous disorders, and vasculitis. It works by inhibiting dihydrofolate reductase and thymidylate synthase, decreasing DNA synthesis and replication. Common side effects include myelotoxicity, hepatotoxicity, and gastrointestinal toxicity. Due to its teratogenic effects, pregnancy is contraindicated and regular monitoring of blood tests is required when taking methotrexate.