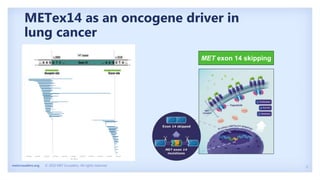
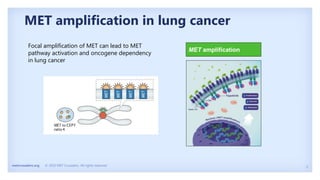
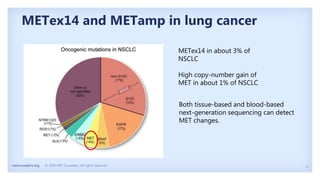
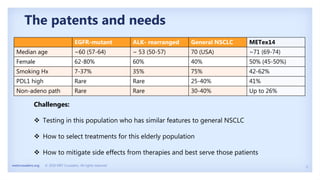
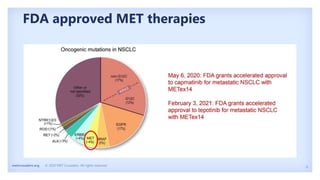
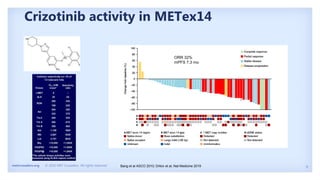
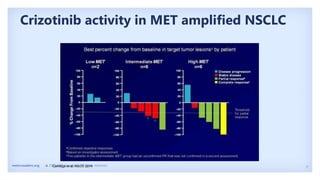
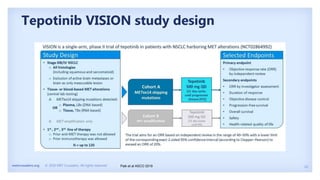
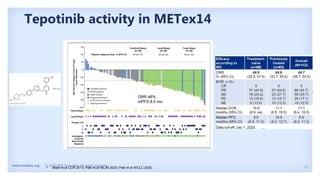
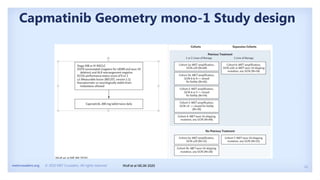
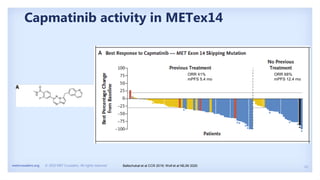
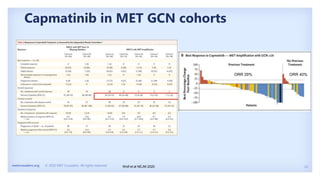
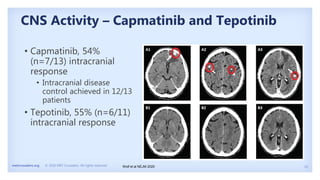
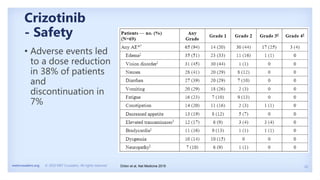
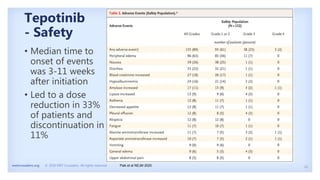
The document discusses new therapies for MET exon 14 (METex14) and MET amplification (METamp) lung cancers, which represent specific oncogenic drivers in non-small cell lung cancer (NSCLC). It highlights the essential role of next-generation sequencing in detecting MET alterations and summarizes the efficacy and safety profiles of various FDA-approved MET inhibitors, including crizotinib, tepotinib, and capmatinib. Challenges in treating this patient population due to age and comorbidities are also addressed, along with considerations for clinical management.