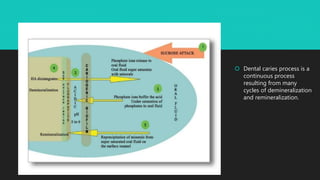
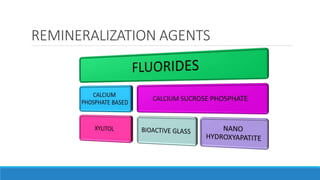
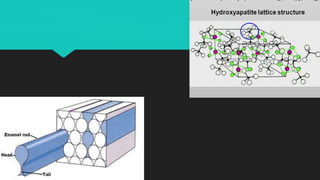
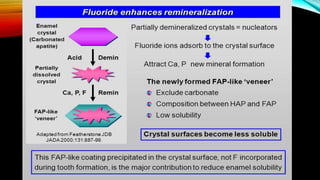
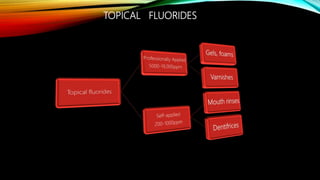
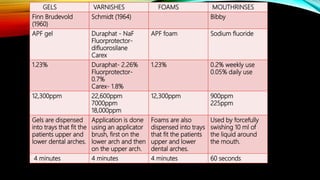
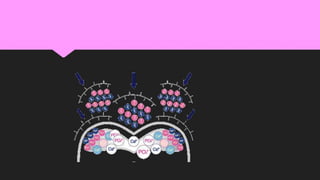
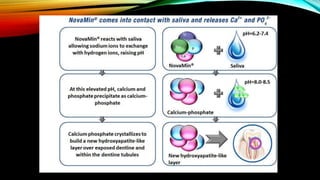
The document discusses the processes of demineralization and remineralization in dental health, highlighting the role of various remineralization agents in combating dental caries. It details the chemical composition of enamel, mechanisms of fluoride action, and various remineralization treatments such as casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) and bioactive glass. Additionally, it covers the clinical applications and benefits of these agents, notably in high-risk patients and specific conditions like enamel erosion and sensitivity.