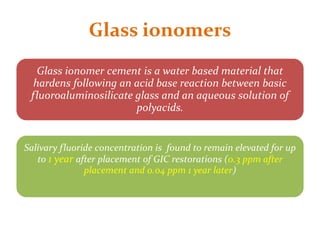
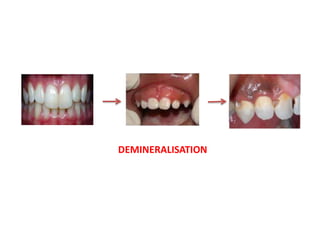
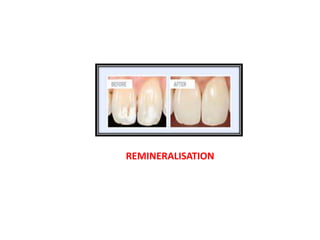
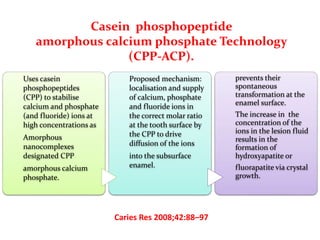
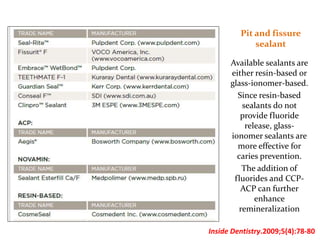
This document discusses various techniques and materials for minimal intervention dentistry and remineralization. It describes the Atraumatic Restorative Technique (ART) which removes decay using hand instruments and restores cavities with adhesive materials. Glass ionomer cements are effective restorative materials for ART due to their fluoride release and adhesion properties. Remineralization involves rebuilding demineralized tooth structure using agents like fluoride and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) which provide calcium, phosphate, and fluoride ions to remineralize enamel. Newer remineralizing systems and delivery methods like dentifrices, sealants, and restorative materials are also discussed.









![REMINERALIZATION
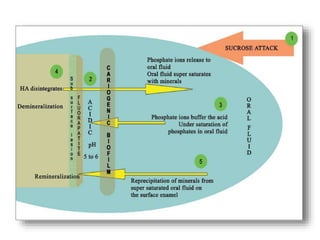
Remineralization is defined as the process whereby calcium and phosphate
ions are supplied from a source external to the tooth to promote ion
deposition into crystal voids in demineralized enamel to produce net mineral
gain
Remineralization of dental lesions requires the presence of partially
demineralized crystals that can grow to their original size when they are
exposed to fluid that is supersaturated with respect to hydroxyapatite
minerals.
• Cochrane NJ, CaiF, Huq NL, Burrow MF, Reynolds EC. New approach to enhance
remineralization of tooth enamel. J Dent Res 2010;89:1187-97.]](https://image.slidesharecdn.com/commonsmr-150920085020-lva1-app6892/85/Remineralization-12-320.jpg)

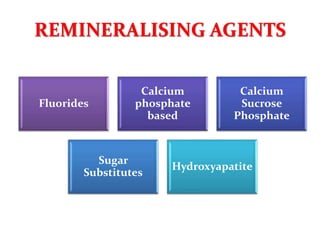








![Clinpro Tooth CrèmeTM
commercially available
organically modified
tricalcium phosphate which
can coexist
with fluoride in aqueous
environment
During brushing,
this toothpaste contacts saliva
and thereby calcium,
phosphate and fluoride ions
become readily available to
tooth thus preventing
demineralization
International Journal of Pharm. Tech Research. 6(2), Apr-Jun 2014,487-493. ]](https://image.slidesharecdn.com/commonsmr-150920085020-lva1-app6892/85/Remineralization-32-320.jpg)



![Dentifrices
One of the most
practical methods
for delivering
remineralizing
agents.
Burwell and Muscle
found that CPP-
ACP provided
sustained condition
for
remineralization
when used in a
dentifrice
J Contemp Dent Pract. 2007;8(7):1-10.]
Dentrifices](https://image.slidesharecdn.com/commonsmr-150920085020-lva1-app6892/85/Remineralization-36-320.jpg)
![Pastes Rinses
and Dental
Floss
.
Pastes used for
remineralization contain
calcium- and phosphate
realeasing components (eg,
CCP-ACP) with or without
fluoride.
Commercial pastes
containing CPP are
designed for professional
application as well as
professionally supervised
home application.
They can be applied via
prophy cup, custom tray,
toothbrush,
or fingertip.
J Dent Hyg. 2008;82(2):19].](https://image.slidesharecdn.com/commonsmr-150920085020-lva1-app6892/85/Remineralization-38-320.jpg)

![In a trial, Manton et al showed that a sugar-free gum containing xylitol produces
superior remineralization.
[Int J Paediatr Dent. 2008;18(4):284-290.]
Sorbitol is another sugar substitute that is used as an artificial sweetener.
Isomalt is a noncariogenic sweetener that is widely used as a sugar
substitute. Adding isomalt to a demineralizing solution has shown to
significantly reduce tooth mineral loss.
[Clin Oral Investig. 2008;12(2):173-177.]](https://image.slidesharecdn.com/commonsmr-150920085020-lva1-app6892/85/Remineralization-40-320.jpg)