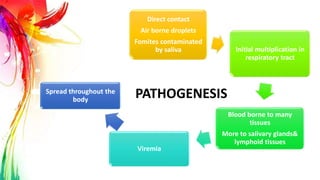
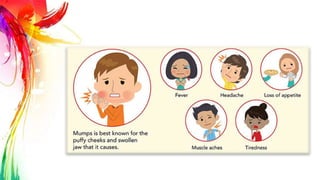
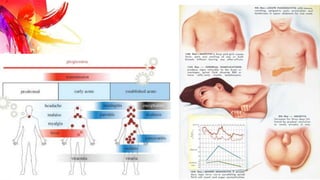
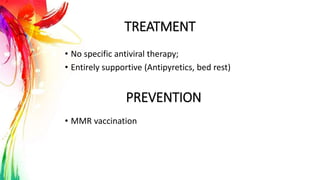
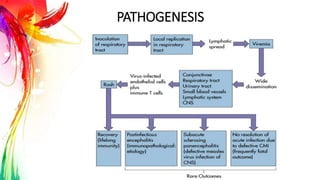
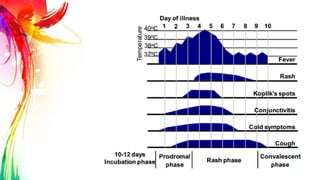
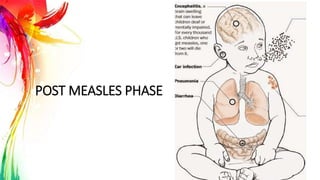
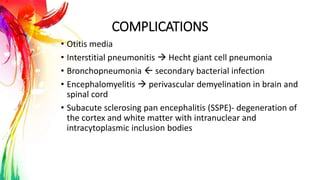
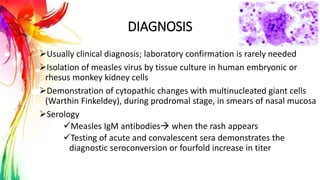
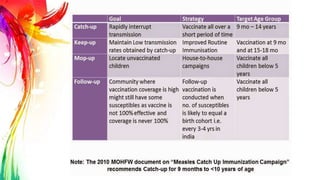
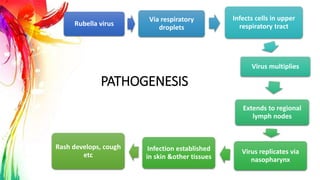
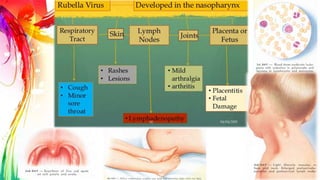
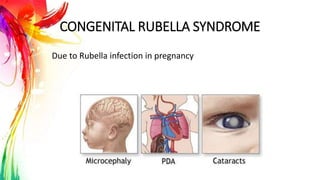
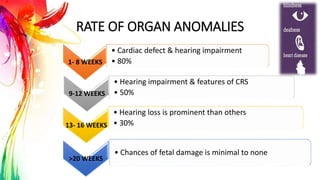
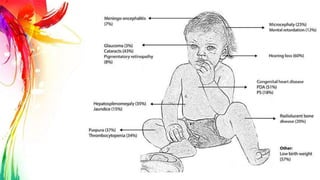
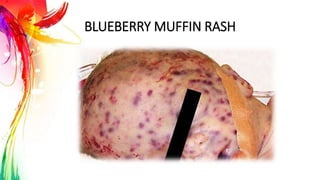
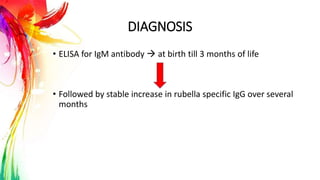
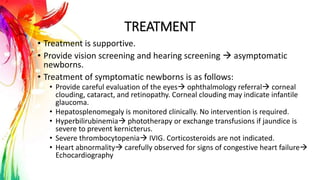
This document provides information on Mumps, Measles, and Rubella. It discusses the causative agents, hosts, environments, pathogenesis, clinical manifestations, diagnosis, treatment, prevention, and vaccination for each disease. Mumps is caused by a paramyxovirus and presents with parotid gland swelling. Measles is caused by a morbillivirus and presents with a rash and Koplik's spots. Rubella is caused by a togavirus and often presents asymptomatically or with mild symptoms and rash. Complications can include encephalitis, deafness, and congenital rubella syndrome. Diagnosis involves virus detection and serology. Treatment is supportive. Prevention relies on vaccination with the