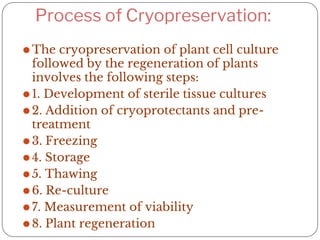
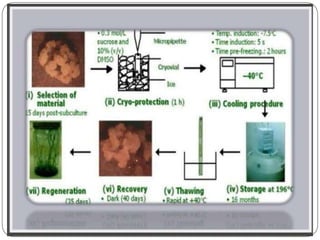
The document outlines the 8 key steps to cryopreserve plant cell cultures:
1. Develop sterile tissue cultures from appropriate plant material
2. Add cryoprotectants like DMSO or glycerol to tissues before freezing
3. Freeze tissues using slow, rapid, or stepwise freezing into liquid nitrogen
4. Store frozen tissues in liquid nitrogen at -196°C for long-term preservation
5. Thaw tissues quickly to avoid ice crystal formation
6. Reculture tissues to remove cryoprotectants
7. Measure viability using staining or regrowth ability
8. Regenerate whole plants from surviving cryopreserved cultures