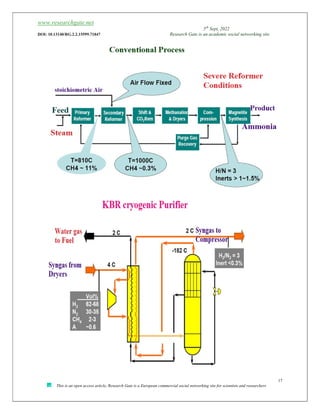
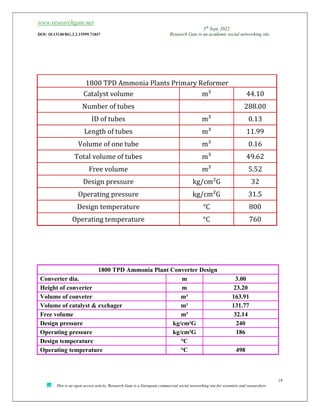
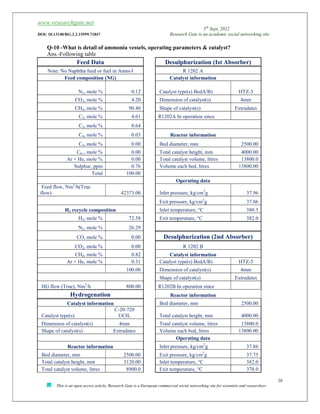
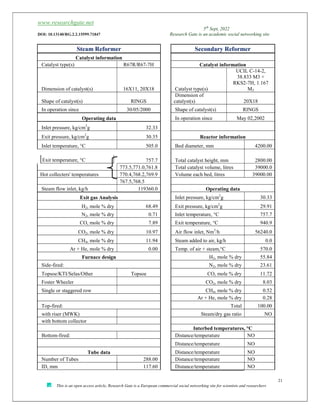
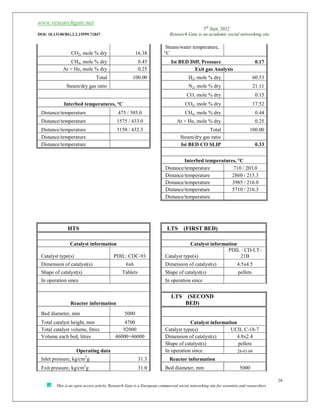
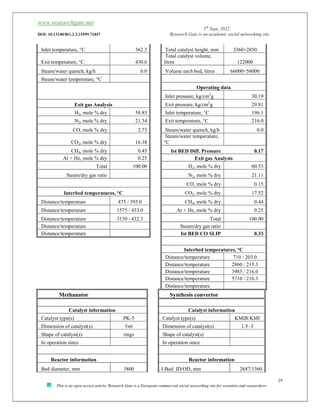
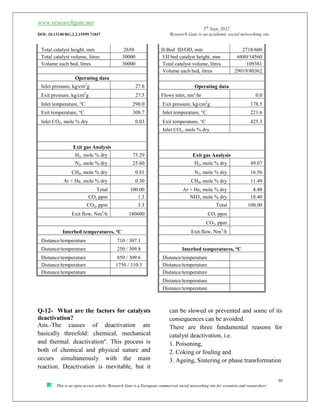
The document discusses ammonia production, focusing on various feedstocks such as natural gas, coal, and naphtha, highlighting the advantages and disadvantages of each. It details the technologies for ammonia production, including significant licensors, and compares conventional processes and modern alternatives for efficiency and environmental impact. The exploration of clean production methods such as electrically heated reforming and the potential future of using coal bed methane are also noted.


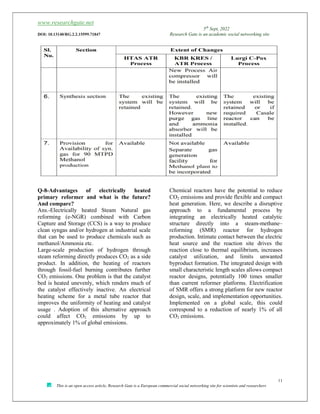
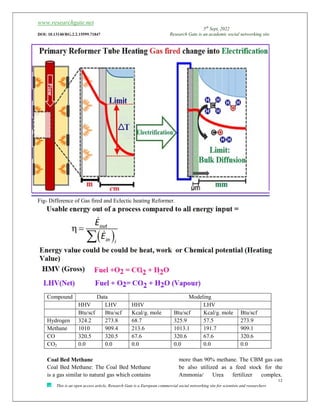


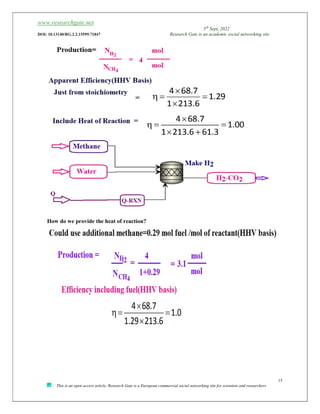
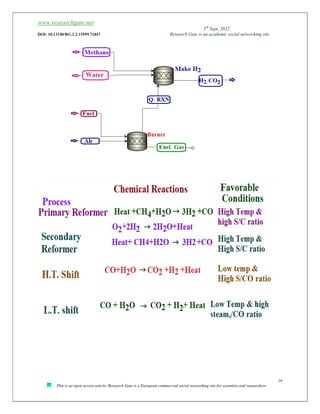







![www.researchgate.net
5th
Sept, 2022
DOI: 10.13140/RG.2.2.15599.71847 Research Gate is an academic social networking site
35
This is an open access article, Research Gate is a European commercial social networking site for scientists and researchers
continuous carbide/precipitate films usually
containing silicide precipitates appears to be
associated with the embrittlement process. The
objective was to study the formation of the
silicides. It is intended that this work will be
completed shortly and reported in a further
paper. Observations at this time suggest that the
solution-annealed material will reform the
complex silicide precipitates with further aging.
Q-25-Will you please list the top critical areas
of ammonia and methanol plants (old plants)
where mix of alloys might be present and
consequently similar failures to this accident
might occur? What kind of inspection tests
and stress analysis are recommended during
plant turnarounds?
Ans.- only ammonia plants, dissimilar weld
areas are seen mainly in the reform furnace
convection section, and Syn Loop section. The
rule of thumb and main emphasis is on austenitic
to ferritic combinations [that is, austenitic to
ferritic materials, such as P1, P4, P5 to P8, P43,
P45] with a pipe wall thickness greater than 12
mm, and an operating temperature of 150 0
C or
above. The preferred method of inspection is
shear wave UT [assuming butt welded
connections] on the ferritic side of the weld on a
minimum two-year interval unless the plant is
cycled, in which case the inspection time is
shortened. Finite Element Analysis is the
preferred stress analysis technique.
Q-26-What changes did you make to your
inspection programs as a result of these
incidents?
Ans.- Agrium had several dissimilar weld
failures shortly after the plant was
commissioned [that is, within one to one and
half years after commissioning]. All dissimilar
weld joints is made [austenitic to ferritic
materials] and these locations have been subject
to inspection by shear wave UT each
turnaround. We have not always been vigilant in
this inspection program and as a consequence
inspections have slipped on occasion, sometimes
culminating in dissimilar weld metal pipe
failures.
Q-27- How was the ammonia converter
isolated from the rest of the Syn Loop to do a
hot work job for weld repair?
Ans.- The exit line from the ammonia converter
to the Syn Loop WHB has an in line valve, and
as well, the bypass line to the ammonia effluent
feed/effluent exchanger [Kellogg design] also
has a valve. These valves were totally removed
and blinds installed. In the case of the blind to
the Syn Loop WHB, the blind has a connection
to bleed in some nitrogen to the Syn Loop WHB
and downstream high-pressure exchangers.
There is an additional blind installed
Downstream of several of the high-pressure
exchangers situated downstream of the
converter. For the ammonia converter per se, a
small nitrogen bleed is let into the converter
through a pre existing connection to preserve
the converter catalyst. The ammonia converter is
depressurized, but not purposely cooled down.
Q-28- What kind of evaluation did you
perform in the equipment downstream of the
converter? Did you find any limitation on
those equipment pieces? What preparation
activities did you perform in advance, in
previous plant turn-around, to ensure the
completion of this modification on the
converter within the actual plant turnaround
schedule?
Ans.- The evaluations that will be carried out on
equipment downstream of the converter included
checking of the equipment design conditions
against the expected operation. Thickness
measurements of ammonia converter
downstream piping can be carried out in one of
the earlier turnarounds to make an accurate
assessment of its condition. No limitations were
identified in the downstream equipment. The
measurements of flange gaps in ammonia
converter outlet piping (in hot and cold
condition) can also be carried out in order to
achieve the same tightness after S-300 basket
installation. Other than the above, there are no
specific preparations that are completed in a
turnaround preceding the one in which the
revamp will be carried out.
Q-29-How does the results of the LOTIS
inspection compare with metallographic
samples in reformer tubes to confirm
remaining life?
Do you plan to include any other NDE
method with the LOTIS system since no one](https://image.slidesharecdn.com/generalknowledgeonammoniaproductionbyprembaboo-220905092114-e6669d38/85/General-Knowledge-on-Ammonia-Production-By-Prem-Baboo-pdf-35-320.jpg)






