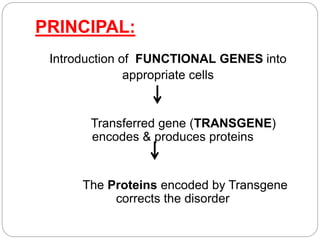
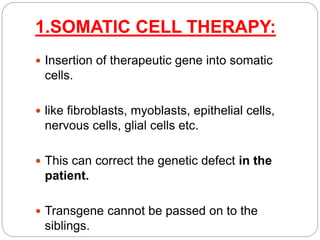
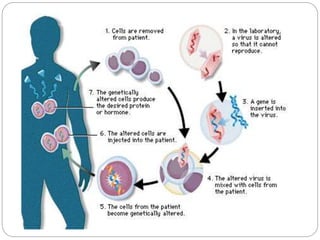
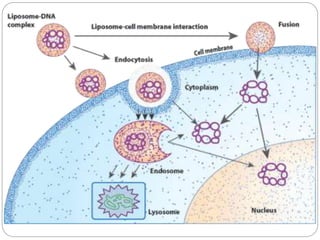
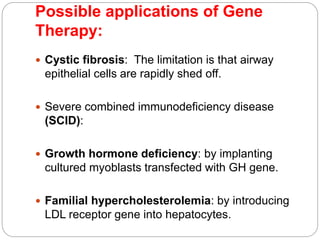
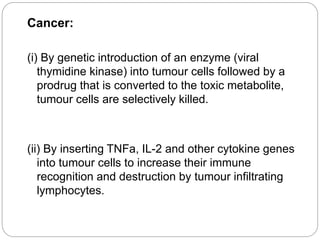
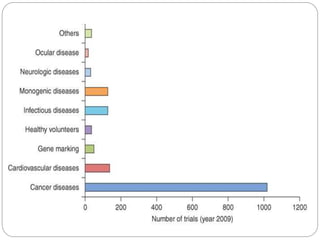
Gene therapy involves inserting a normal gene into a patient's cells to replace a defective gene that causes disease. There are two main types of gene therapy: somatic cell therapy, which treats the individual patient, and germline therapy, which could affect future generations. The two most common methods of delivery are ex vivo therapy, where cells are removed and modified before being returned, and in vivo therapy through direct injection. Retroviruses and adenoviruses are the most widely used vectors for transporting genes, but each has advantages and disadvantages for successful gene expression and integration. While promising for treating many diseases, gene therapy still faces challenges from issues like gene silencing, immunogenicity, and potential safety risks that require more research.