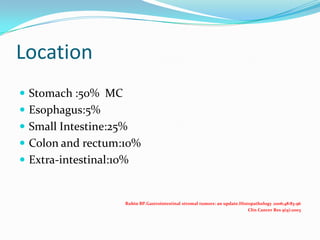
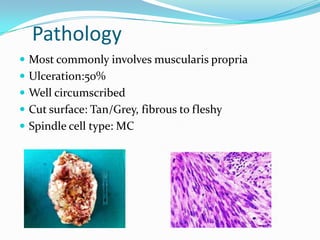
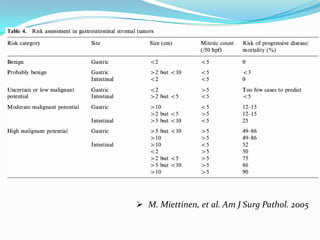
Gastrointestinal stromal tumors (GISTs) are rare tumors that originate from the gastrointestinal tract. They most commonly occur in the stomach. Surgical resection is the primary treatment, but molecular targeted therapy with imatinib is also used in advanced or unresectable cases. Imatinib has improved outcomes by reducing recurrence rates after surgery or controlling tumor growth. Ongoing clinical trials are further evaluating the neoadjuvant and adjuvant uses of imatinib to improve prognosis.