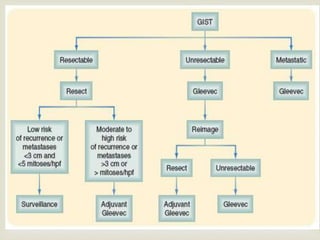
GIST are rare mesenchymal tumors that arise from interstitial cells of Cajal in the gastrointestinal tract. They commonly express the receptor tyrosine kinases KIT or PDGFRA, which are often mutated. Complete surgical resection is the main treatment for localized primary GIST, while targeted therapy with imatinib or sunitinib is used for advanced or metastatic disease. Factors like tumor size, mitotic rate, and site of origin determine prognosis and risk of recurrence to guide adjuvant targeted therapy or surveillance after surgery.