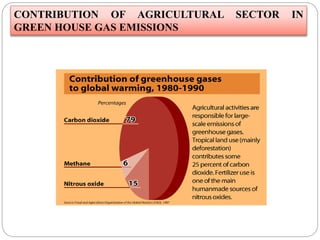
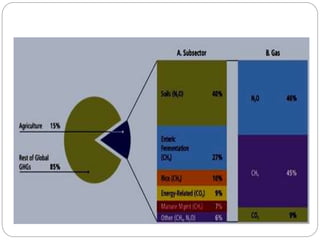
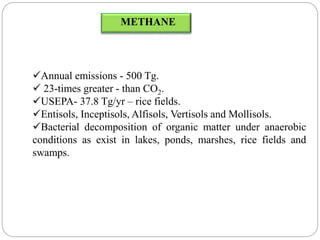
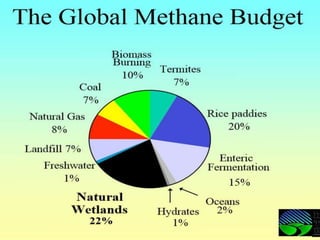
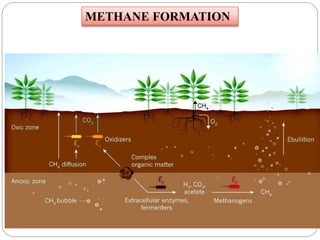
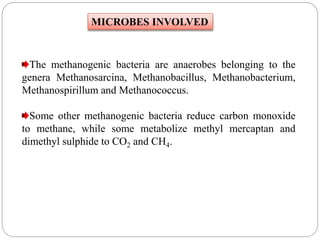
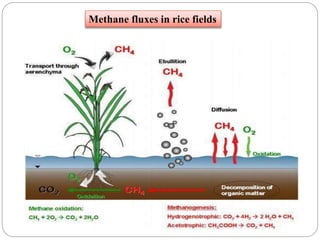
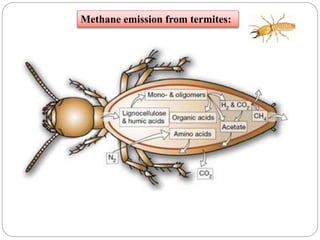
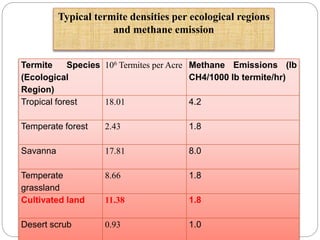
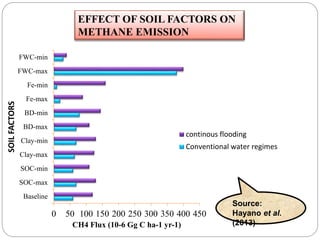
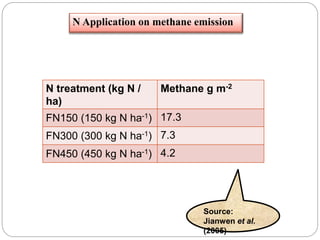
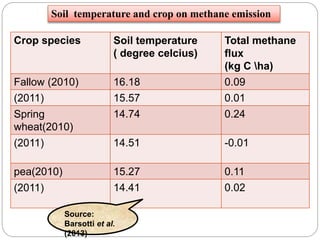
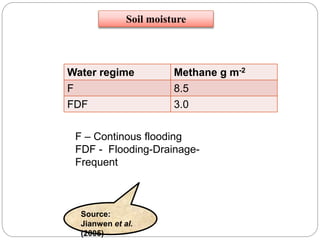
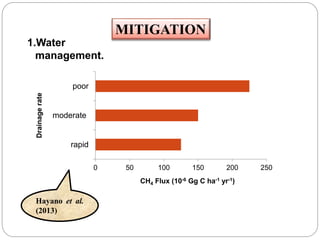
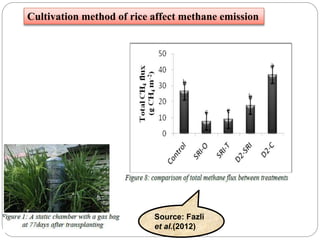
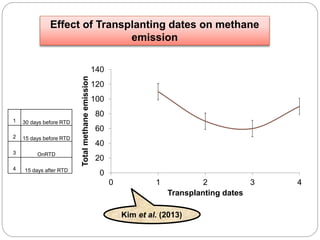
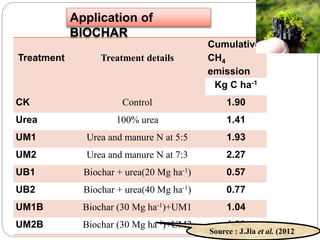
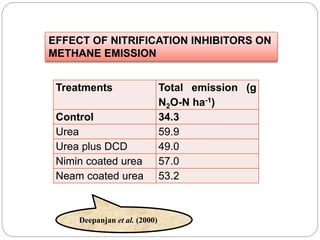
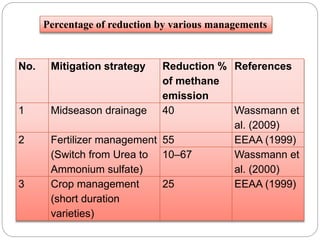
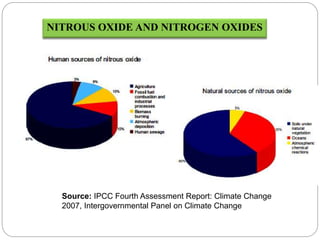
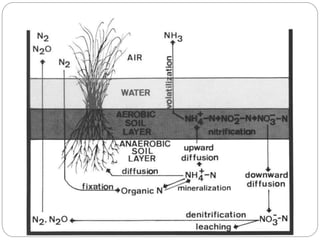
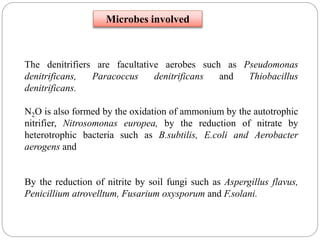
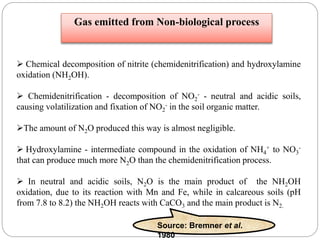
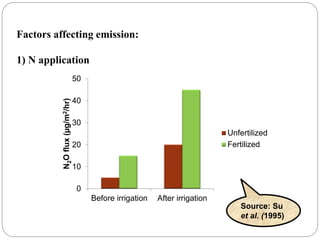
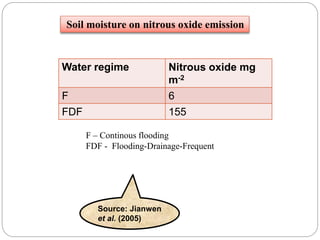
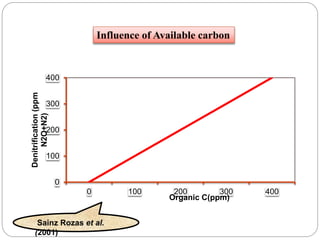
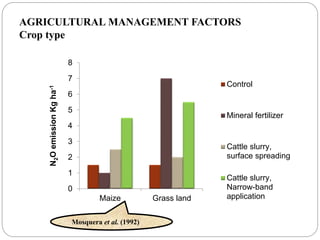
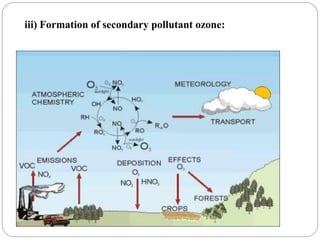
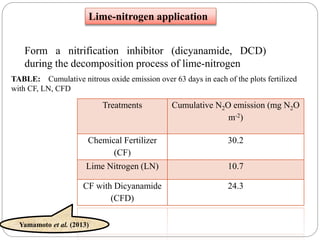
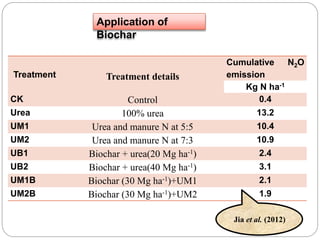
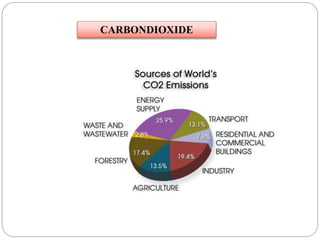
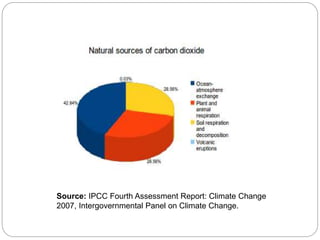
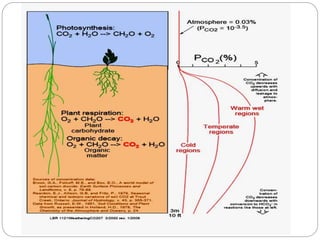
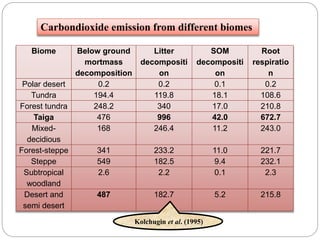
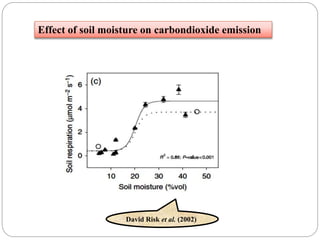
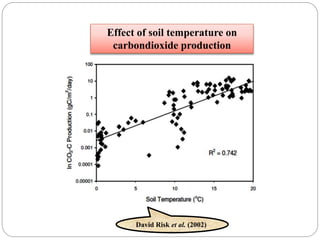
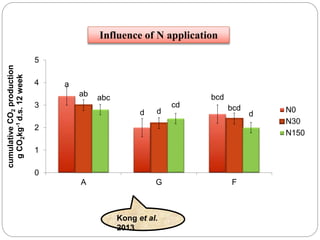
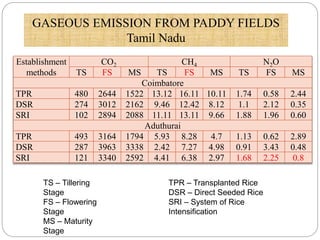
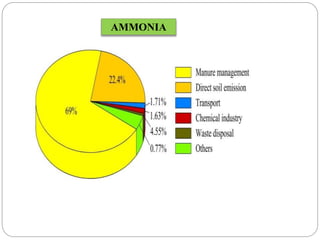
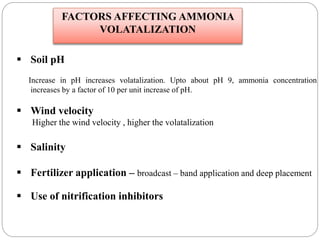
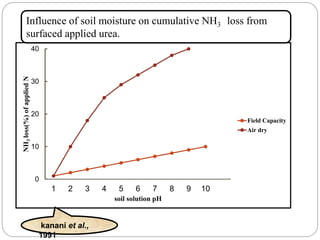
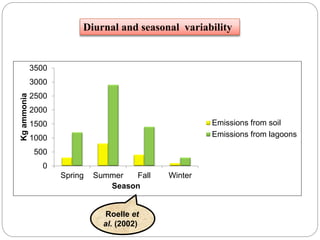
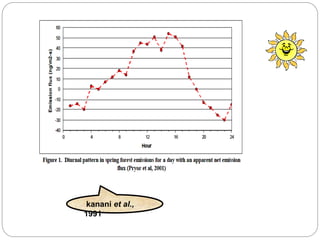
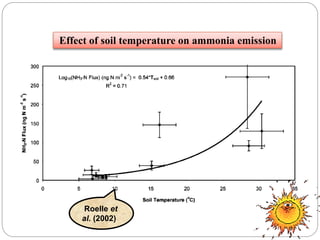
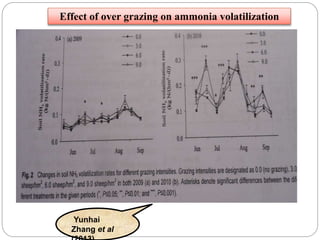
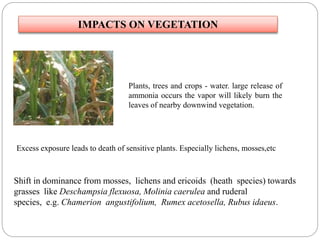
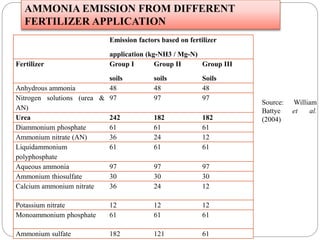
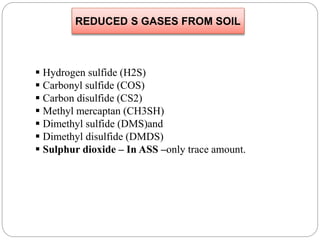
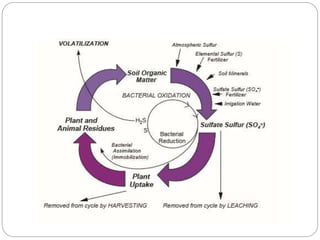
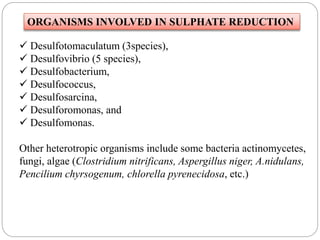
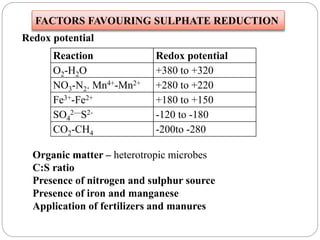
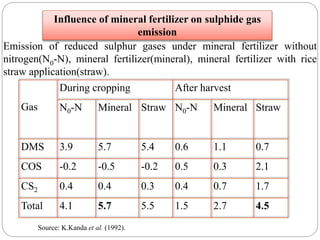
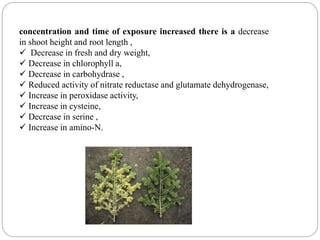
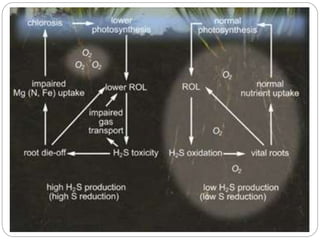
The document discusses the impact of gaseous emissions from agricultural fields, including methane and nitrous oxide, which significantly contribute to greenhouse gas emissions and global warming. Factors affecting these emissions include soil type, water management, and fertilization practices, with various mitigation strategies highlighted to reduce gas emissions. Specific studies and data on methane fluxes, agricultural practices, and the role of different gases are provided to illustrate the issue's complexity and the need for effective management approaches.