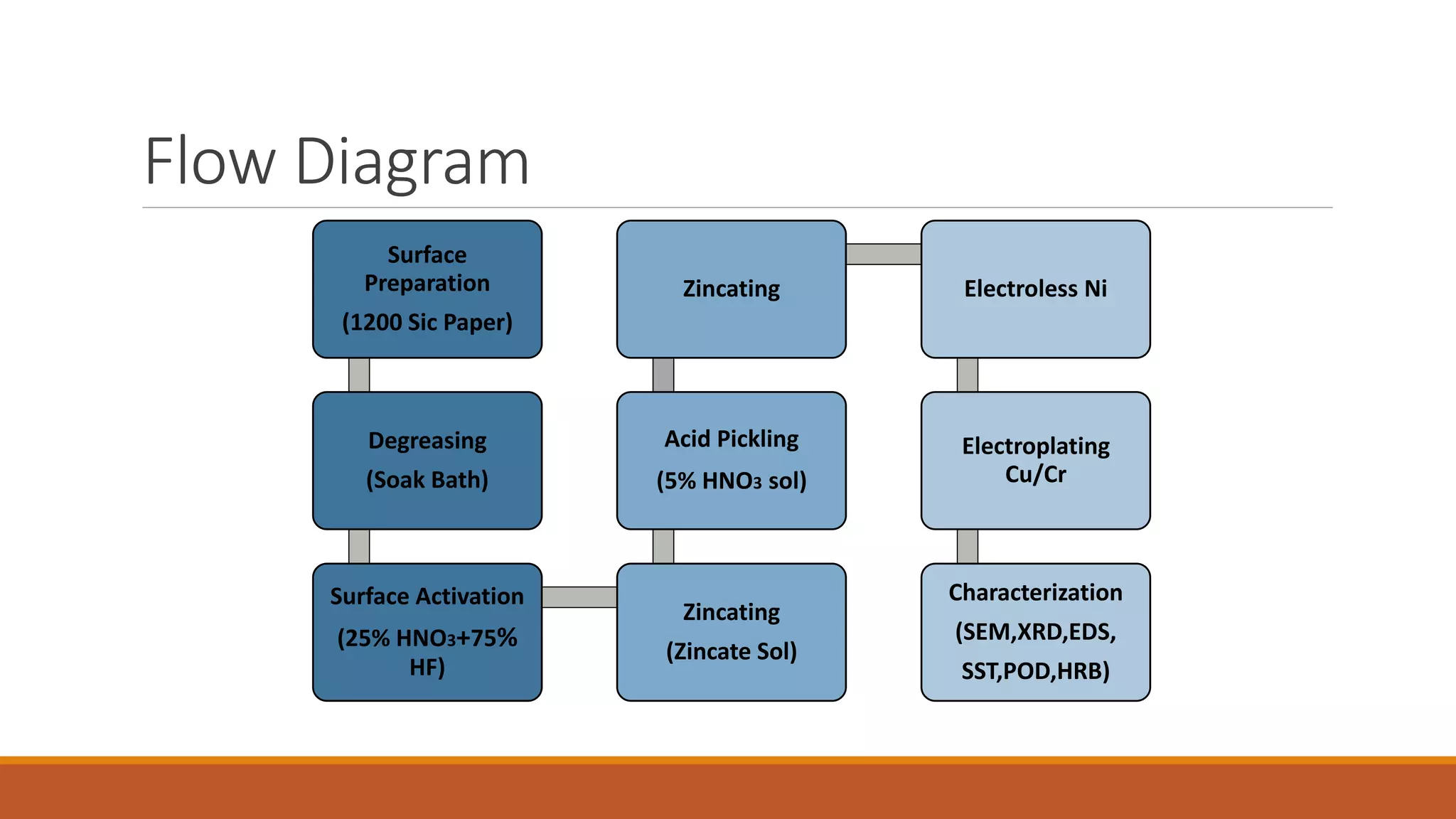
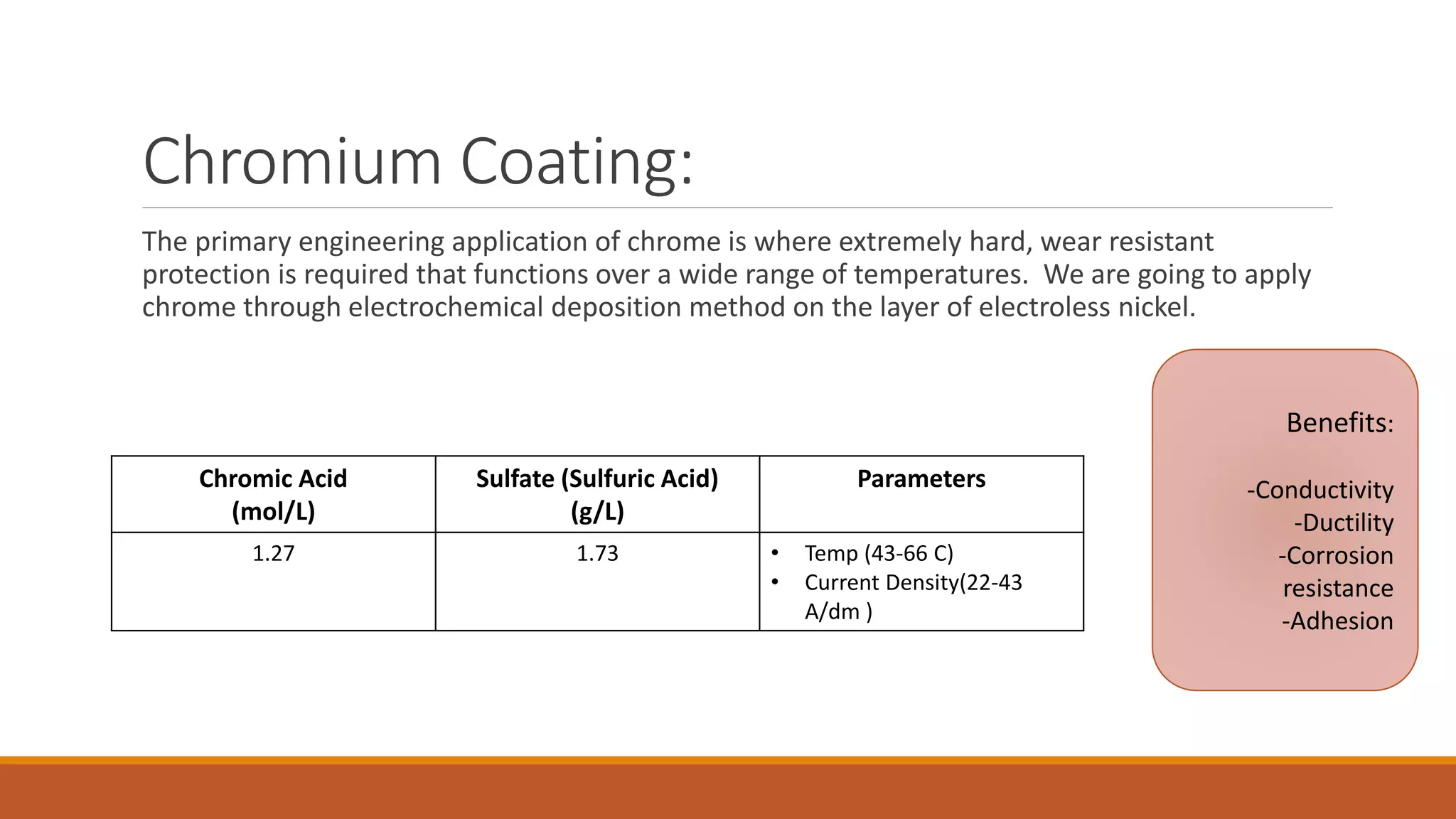
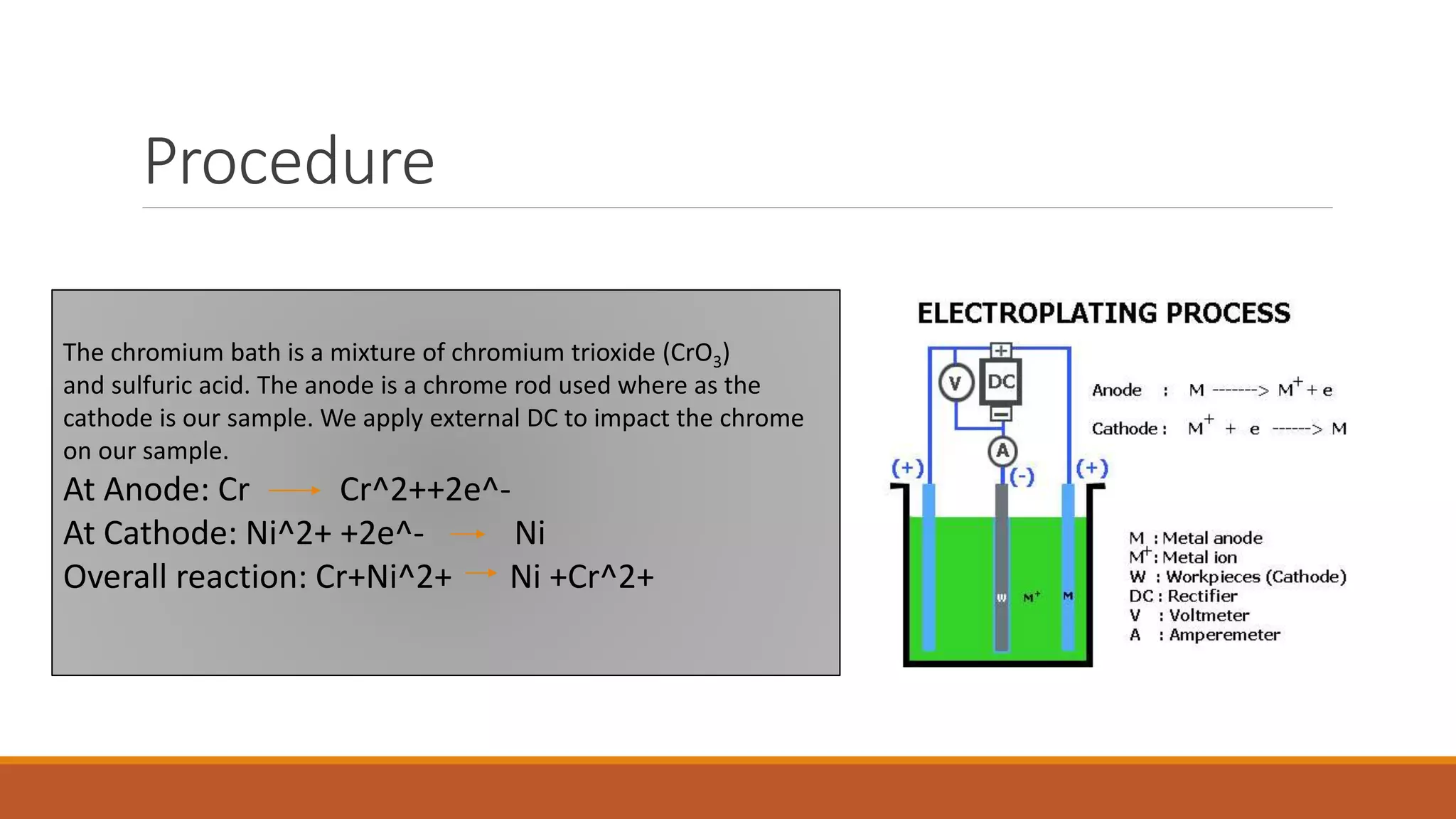
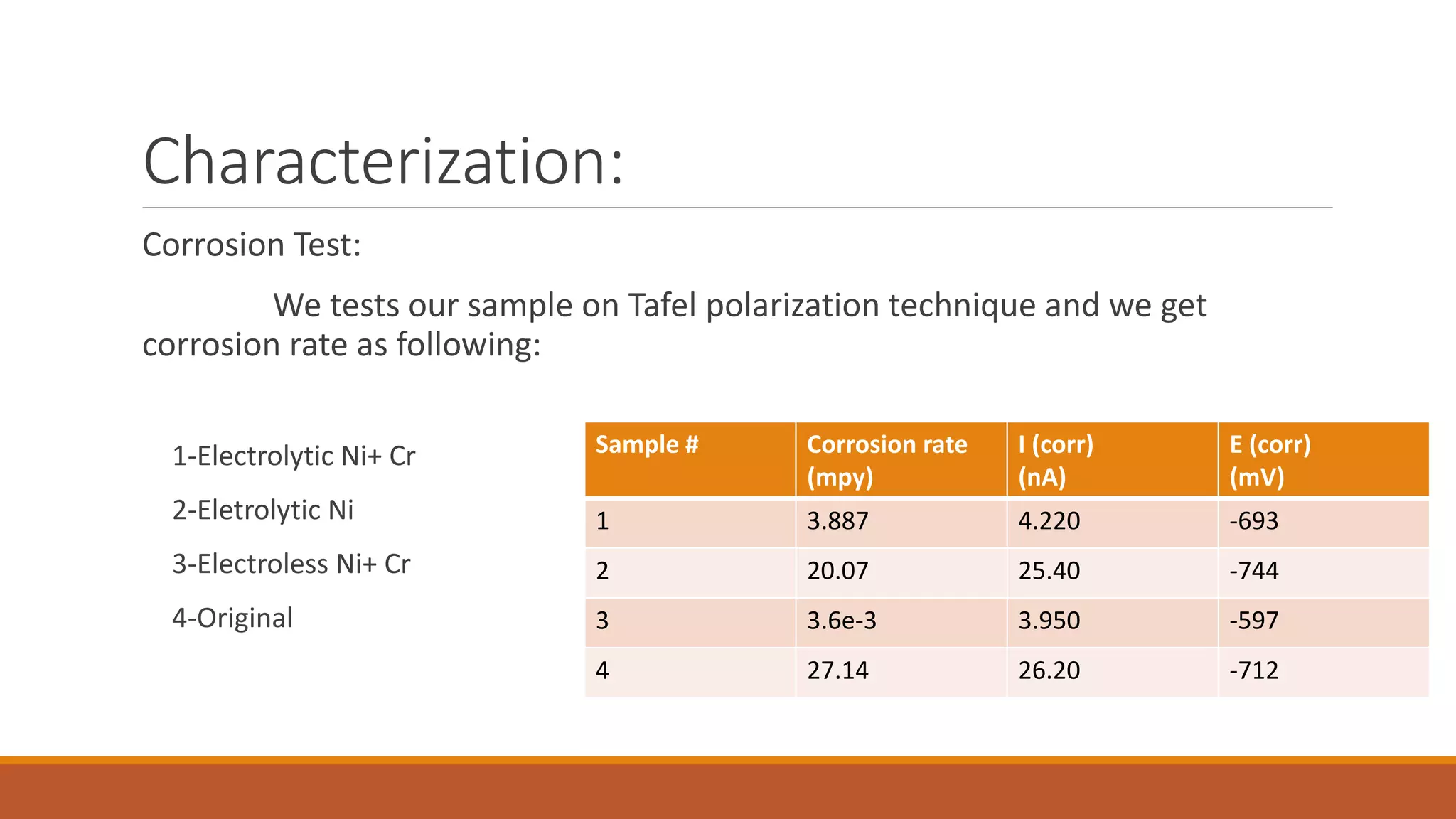
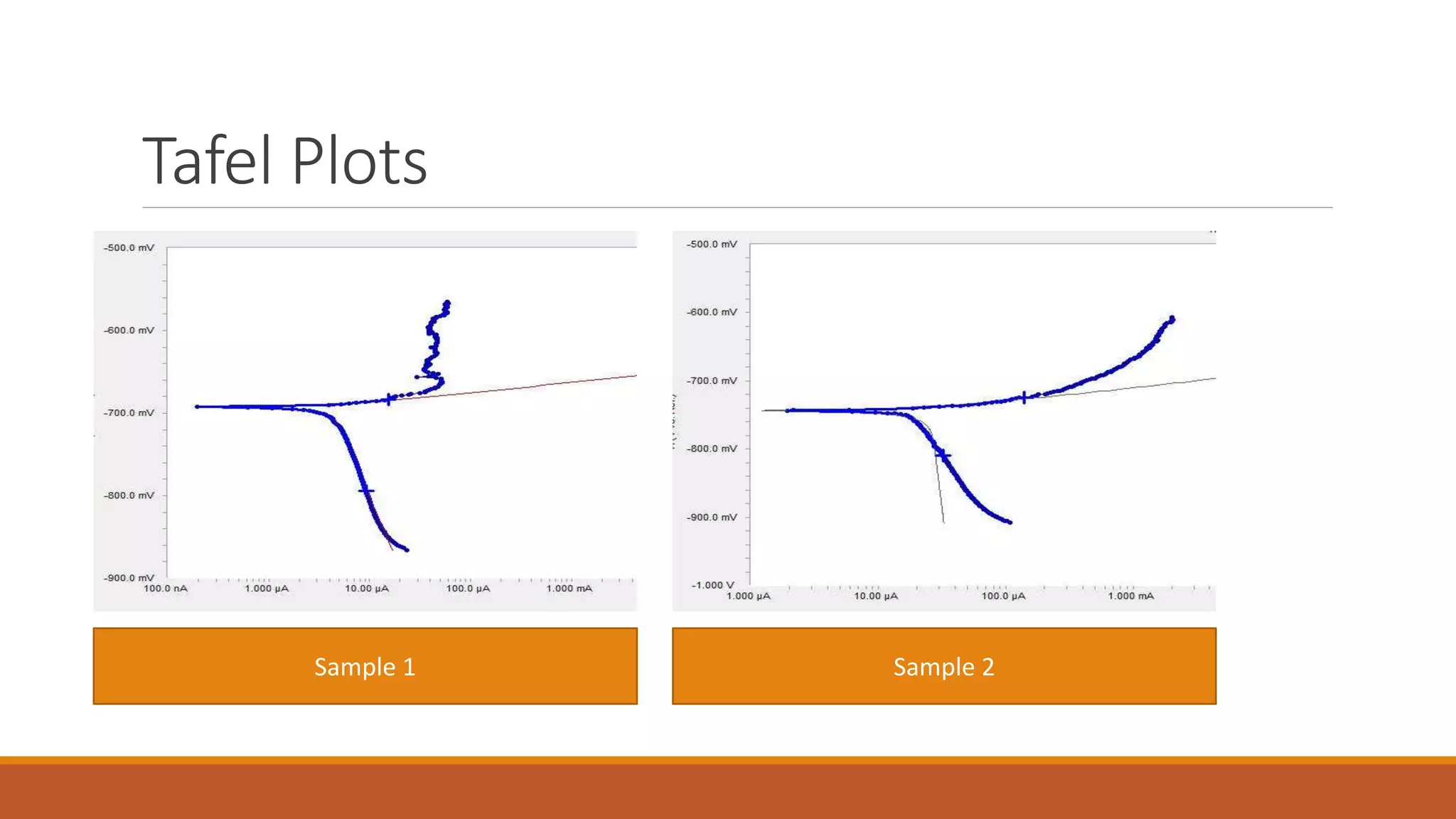
The document describes developing a multilayer coating of electroless nickel and electrolytic chromium/copper on an aluminum 7075 substrate. The coating is intended to improve adhesion, wear resistance, conductivity, and corrosion resistance while achieving a thickness of 10 microns. The coating process involves surface preparation including degreasing, activation, and zincating followed by electroless nickel deposition and electrolytic chromium plating. Characterization of coated and uncoated samples found that the coated sample had better corrosion resistance, hardness, and conductivity. Some issues with reproducibility were identified for further improvement.