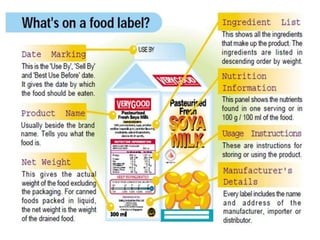
The document discusses food labelling from national and international perspectives. It provides details on:
1) Codex Alimentarius and WHO guidelines for food labelling that many countries use as a basis for their policies.
2) The key elements required on European Union food labels including product name, ingredients, allergens, nutrition information, and business details.
3) The agencies that regulate food labelling in the US depending on the food type, and the mandatory elements required on US labels.