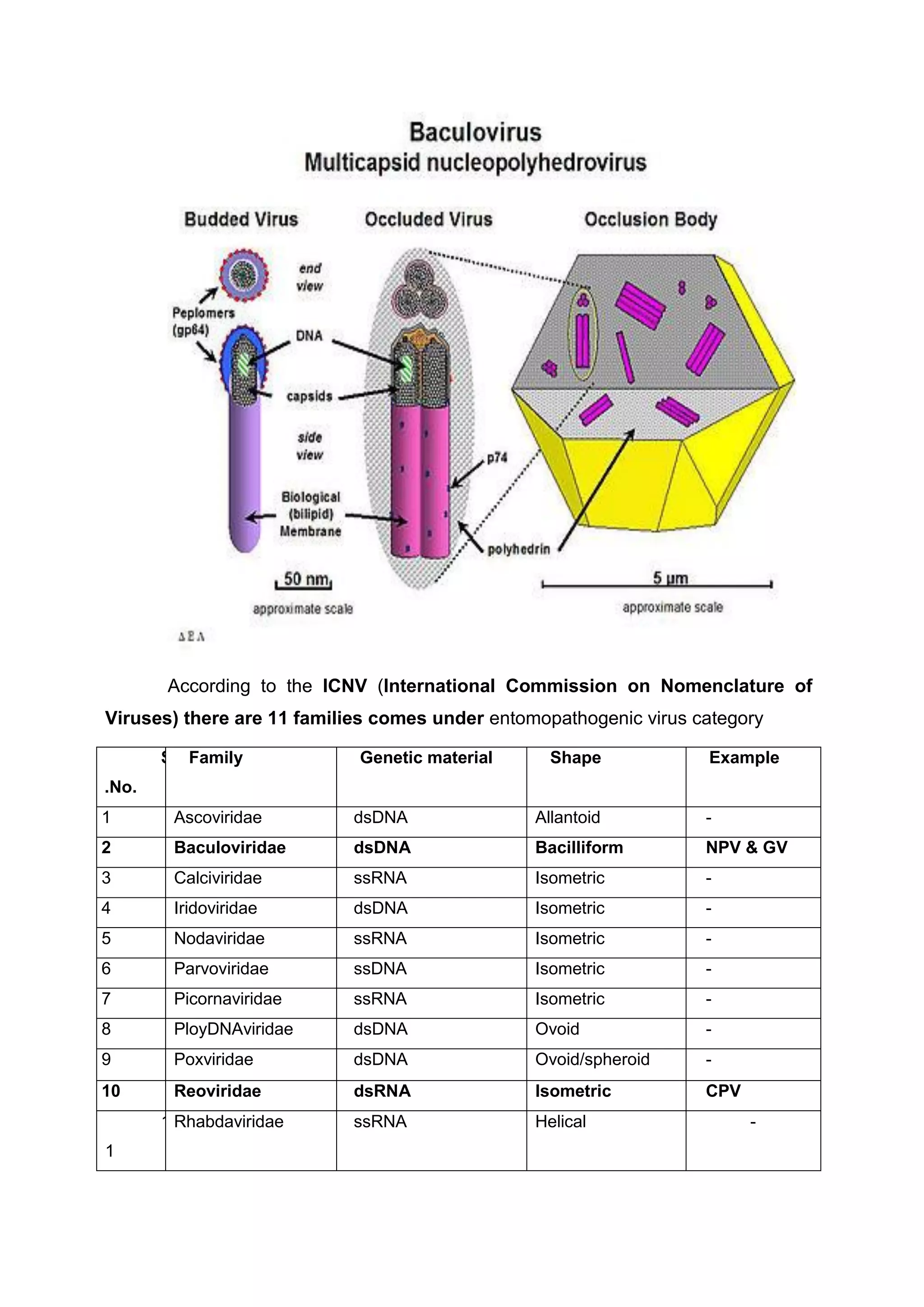
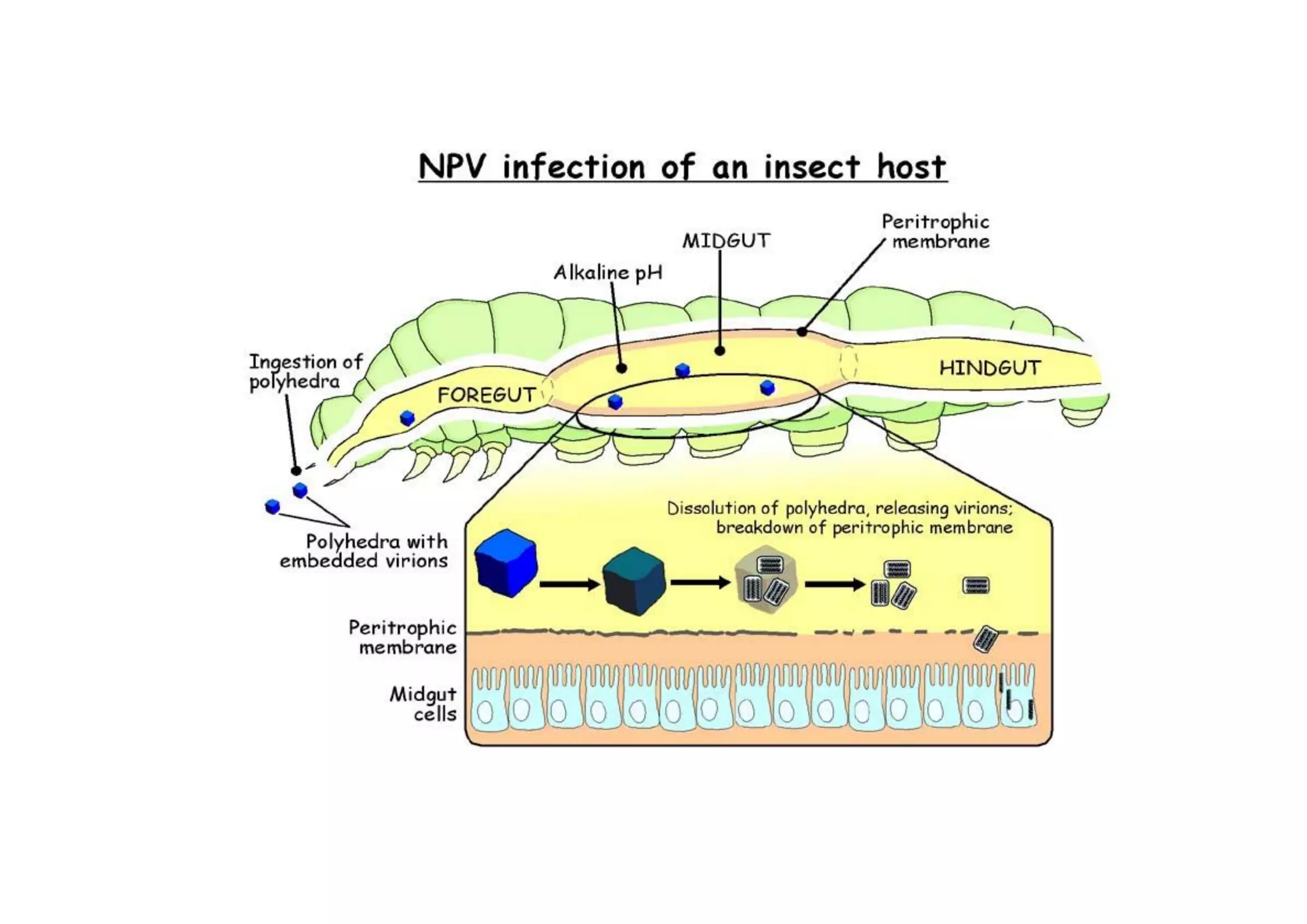
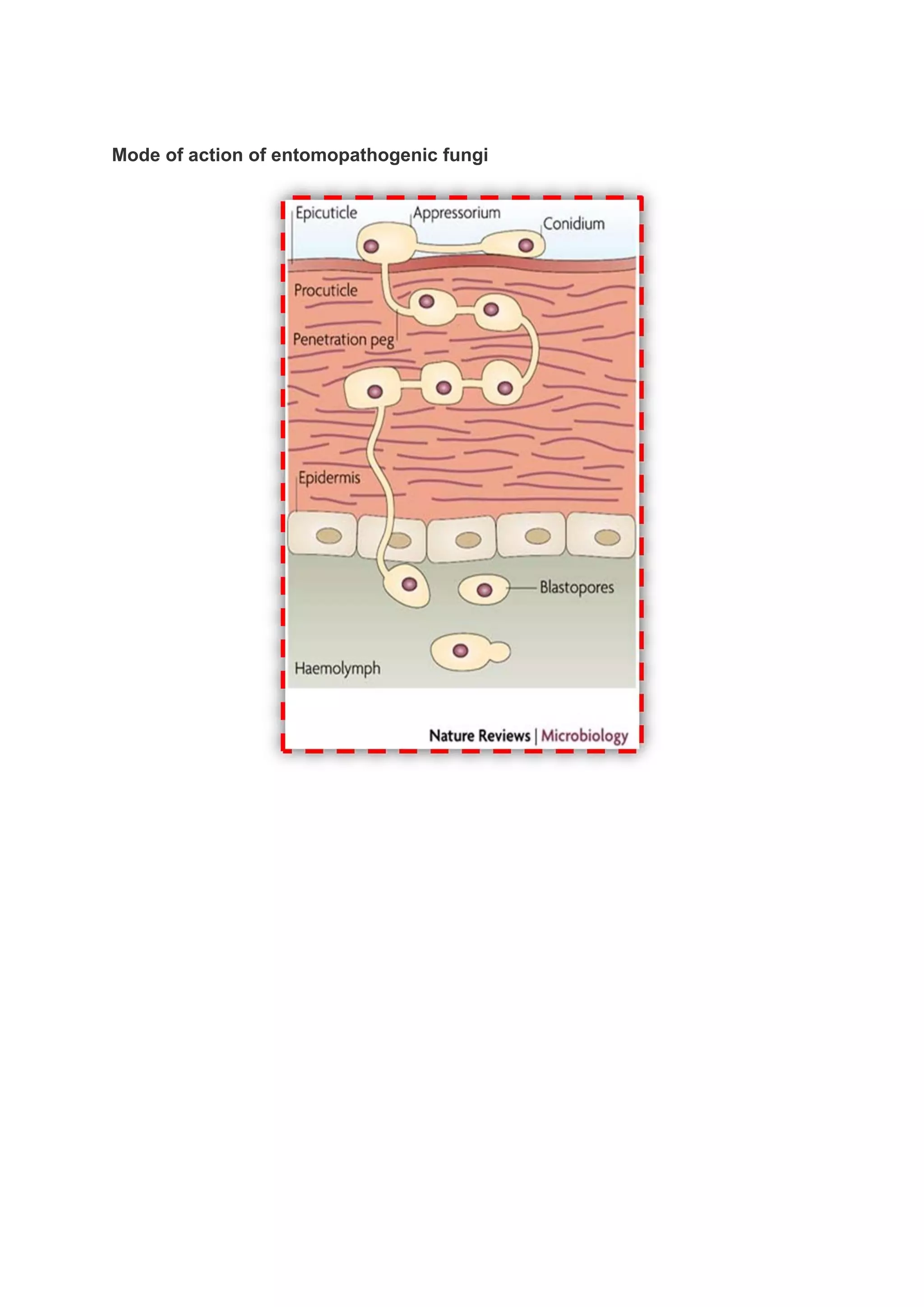
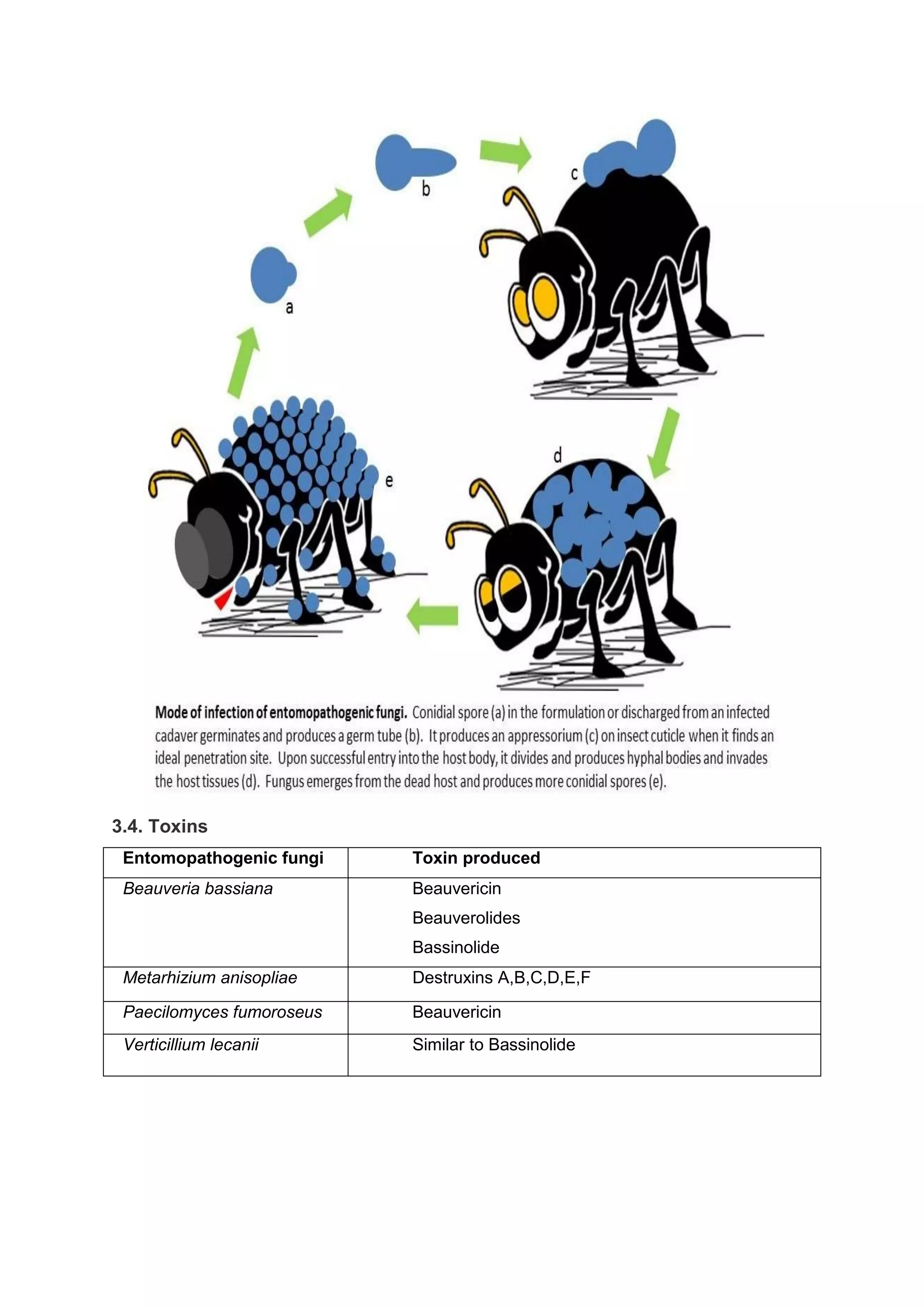
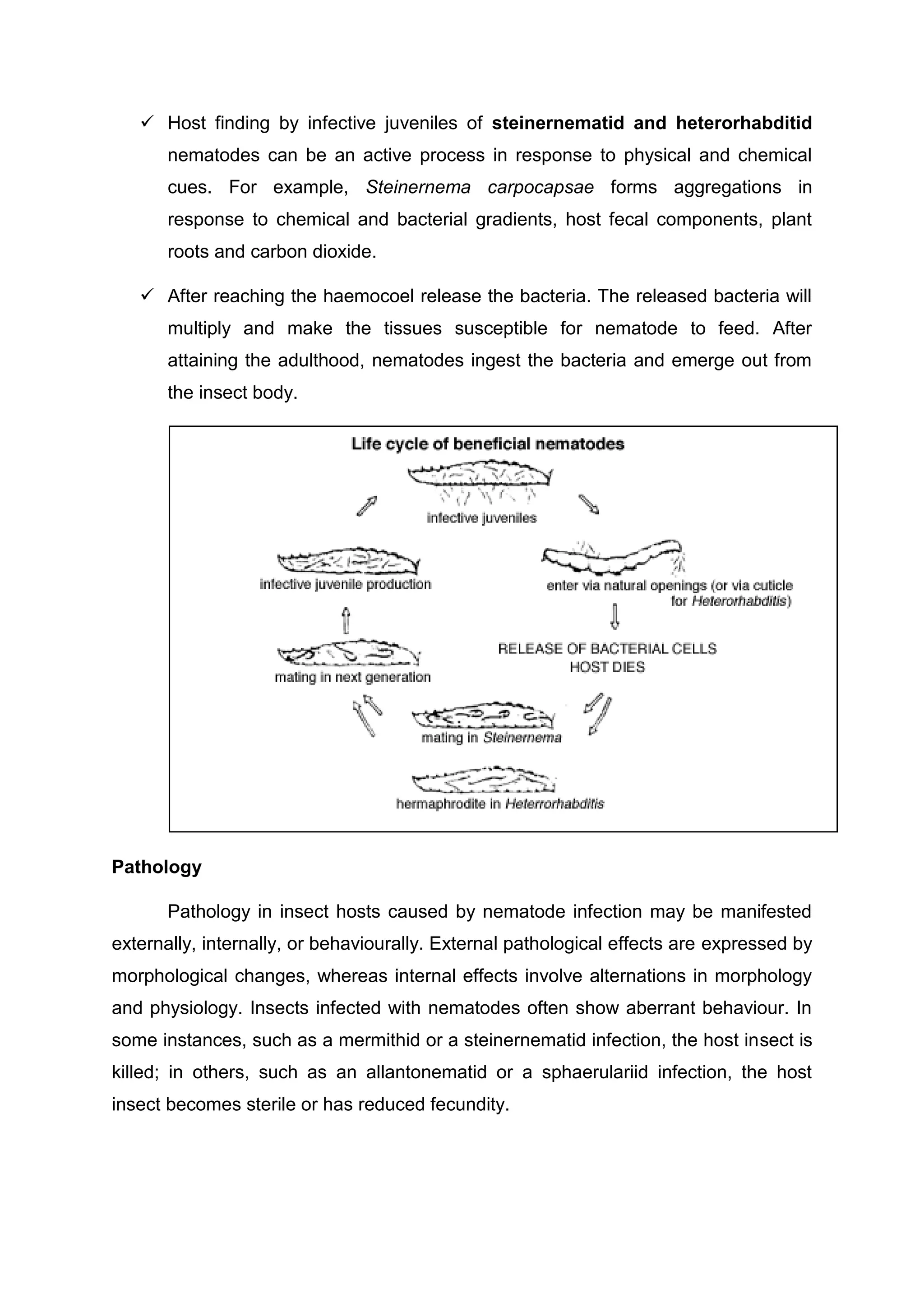
This document provides an overview of biopesticides and their classification. It defines biopesticides according to the USEPA and European Union as naturally occurring substances or microorganisms that control pests. It then classifies common biopesticide types as insect viruses, bacteria, entomopathogenic fungi, entomopathogenic nematodes, and other microorganisms. Specifically, it outlines commonly used insect viruses like NPV and GV, the bacteria Bacillus thuringiensis, and fungal agents like Beauveria bassiana and Metarhizium anisopliae.




![LECTURE NO: 02
INTRODUCTION, DEFINITIONS, TERMINOLOGY, IMPORTANCE, SCOPE AND
POTENTIAL OF BIOPESTICIDES
================================================================
Definitions
1. According to USEPA(United States Environmental Protection Agents :
Biopesticides may be defined as naturally occurring substances that control
pests (Biochemical pesticides), Microorganisms that control the
pests(Microbial pests) and Pesticidal substances produced by plants
containing added genetic material (Plant Incorporated protectants)
2. According to European Union: Biopesticides have been defined as form of
pesticide based on microorganisms/natural products
Terminology
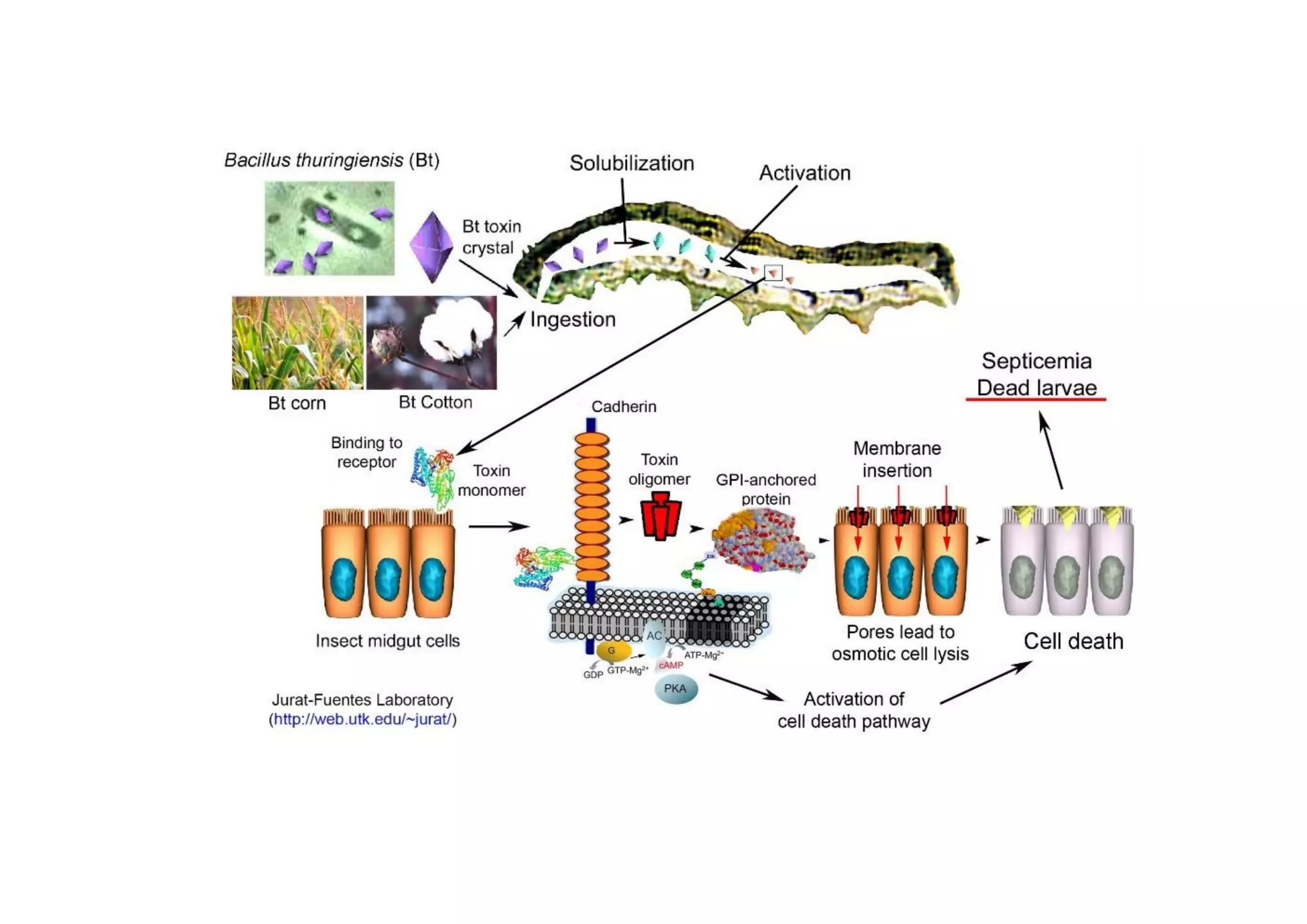
Entomopathogen/ Insect pathogen: Entomopathogens are infectious
agents, microorganisms that invade and reproduce in an insect and spread to
infect other insects. Eg: Fungi, bacteria, actinomycetes and nematodes etc.
Insect pathology: Insect pathology is the study of anything that goes wrong
[i.e., disease (“lack of ease”)] with an insect.
Infectivity: Ability of microorganism to enter the body of a susceptible insect
and produce an infection
Pathogenicity: The quality or state or being pathogenic, the potential or
ability to produce disease
Virulence: The disease producing power of an organism, the degree of
pathogenicity within a group or species
Dosage: A minimal number of infective propagules is needed to pass through
the portal of entry for infection to occur in an insect
Sign: Physical or structural abnormality in an insect as a result of infection.
Eg: abnormalities in the morphology or structure such as colour, malformed
appendages or body segments, fragility of the integument, etc.](https://image.slidesharecdn.com/finalstudymaterialelec230-220416133848/75/Final-Study-Material-ELEC-230-pdf-5-2048.jpg)

































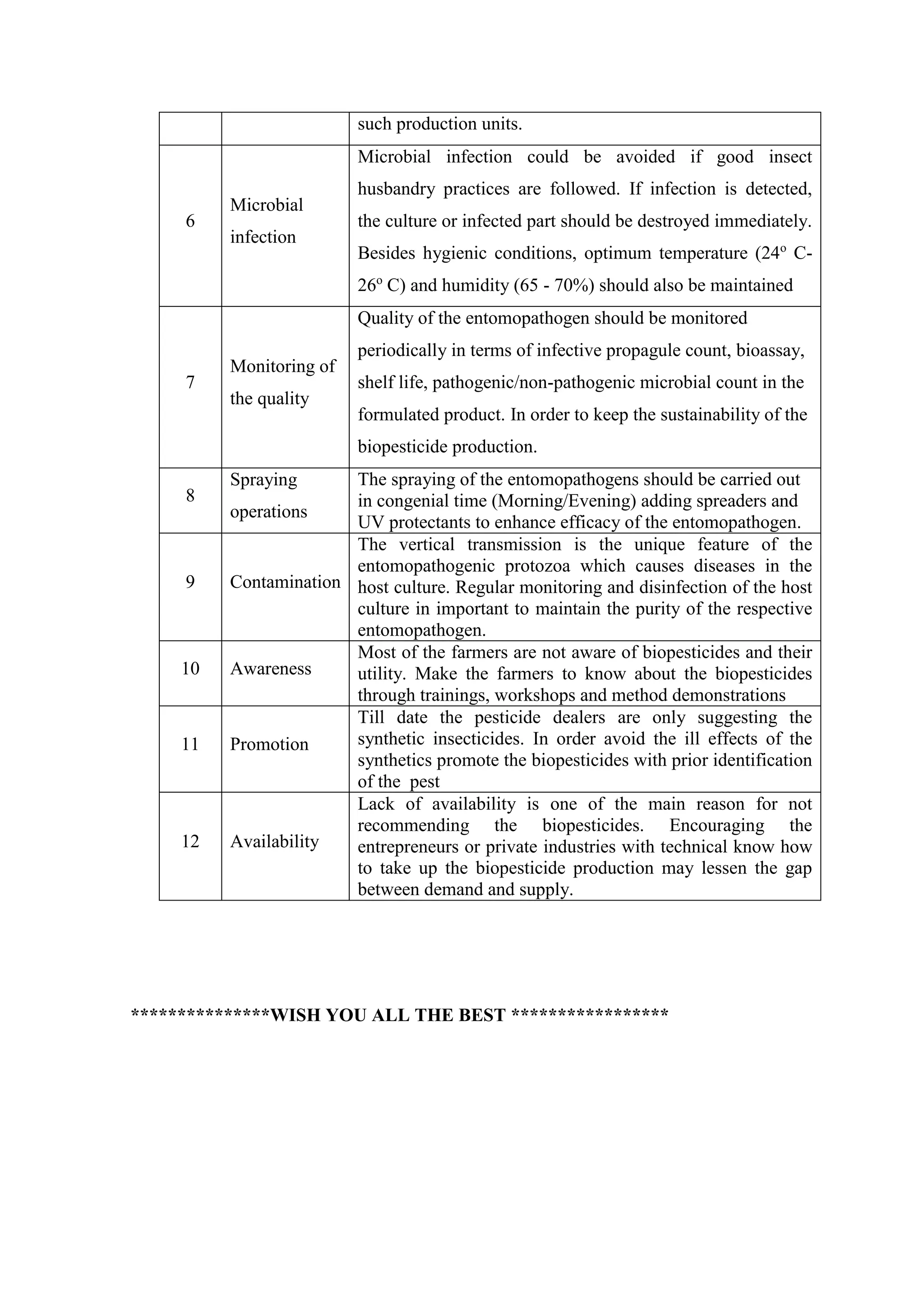
![Mass culturing techniques
The growth of most commonly used entomopathogenic bacteria, B.
thuringiensis and B. sphaericus. Is typically done at 30 ºC and UG medium has
provided reliable and reproducible growth, sporulation, and production of parasporal
bodies in both cases.
Preparation of a 10-ml preculture
From a stock or a colony from a fresh plate, is inoculated into the tube
containing 10 ml UG medium to serve as a preculture.
After inoculation, culture is incubated on a shaker for 48 h at 30ºC. and then
observed for sporulation. After sporulation occurs, the preculture is heat-
treated at 80 ºC. for 10 minutes to kill vegetative cells. Heat treatment allows
for a more consistent growth of the new culture. This preculture will be used to
inoculate the cultures for large scale production.
Harvesting of spores/crystals
Erlenmeyer flask (1 litre) containing 100 ml UG medium is subjected to
sterilization at 121ºC for 15 minutes. After sterilization, glucose is added to
give 1%final concentration. [Glucose stock solution of 10% that has been filter
sterilized should be used]
Flask is inoculated with ~ 0.5 ml of a preculture and incubated at 30º C with
orbital agitation for 48 to 72 h until cell lysis is complete. Culture should be
checked under a phase-contrast microscope to monitor cell lysis, the
sporulation rate and presence of parasporal crystal proteins.
Then culture is subjected to centrifugation at 5000 rpm for 15 to 20 minutes.
supernatant is decanted and pellet of spores and crystals is collected
The pellet of spores and crystals is resuspended with 0.5 M NaCl for 15 min
to avoid exoprotease activity.
The resuspended spore crystal mix is centrifuged at 5000 rpm for 15 to 20
min. Again the pellet is resuspended in distilled or deionized water and
centrifugation is repeated.
Finally, the pellet is resuspended in a water volume identical to the initial
culture (i.e. 200 ml). This material can be used in bioassays to determine](https://image.slidesharecdn.com/finalstudymaterialelec230-220416133848/75/Final-Study-Material-ELEC-230-pdf-60-2048.jpg)