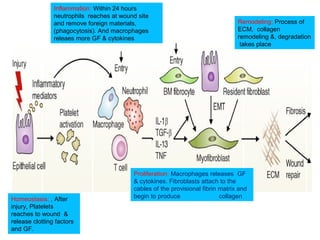
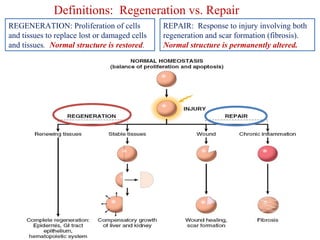
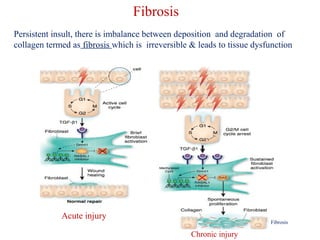
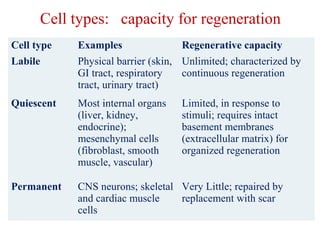
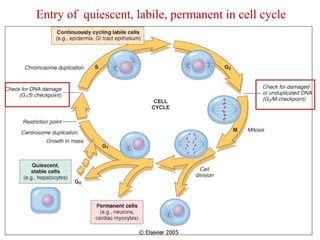
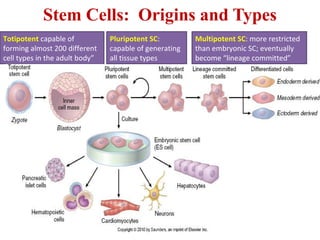
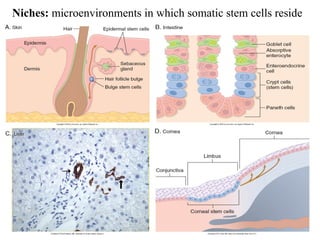
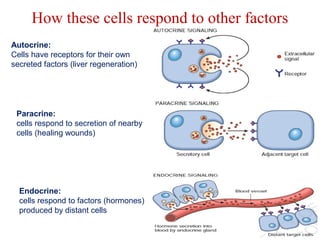
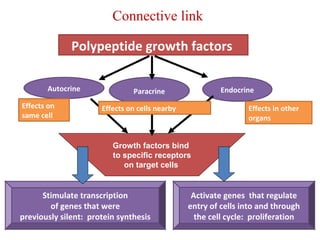
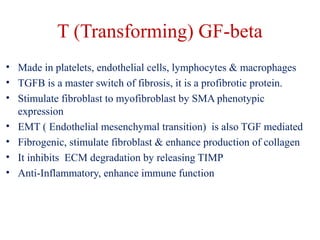
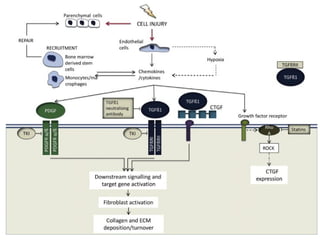
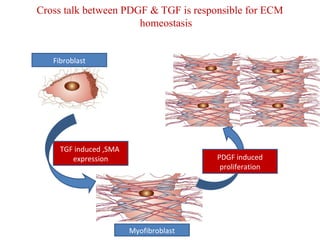
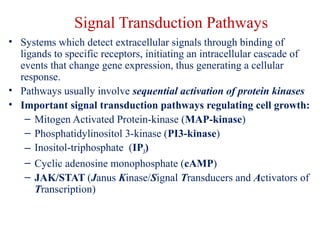
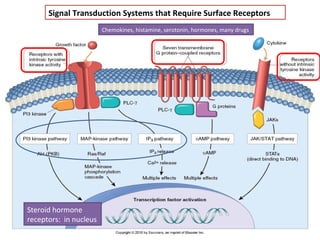
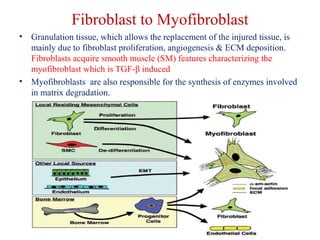
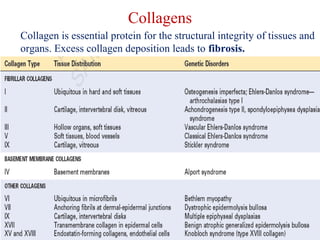
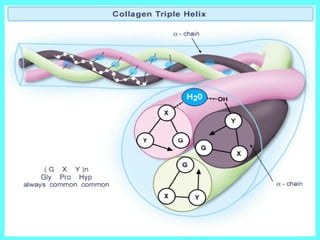
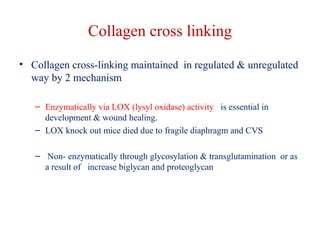
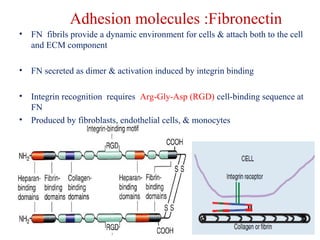
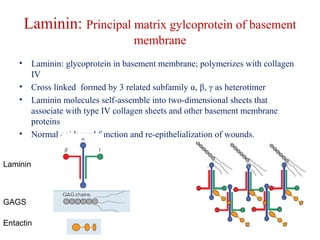
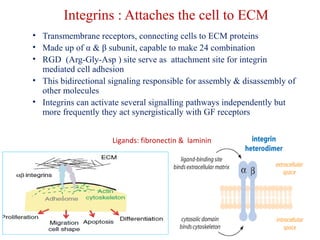
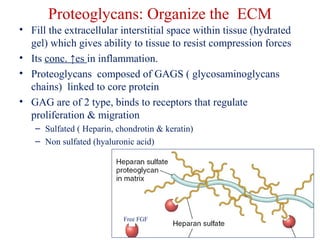
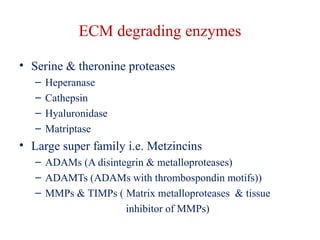
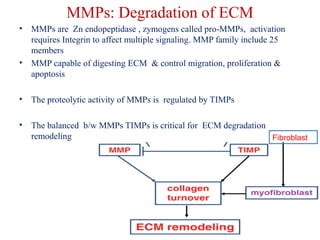
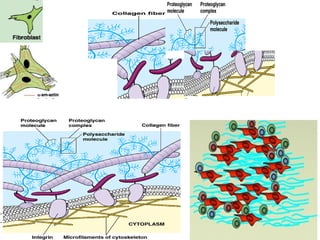
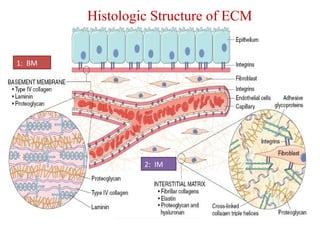
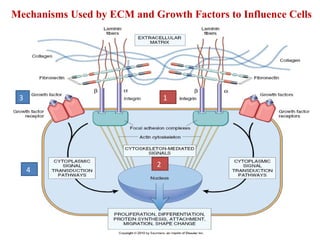
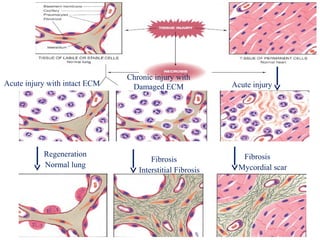
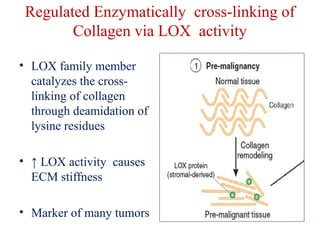
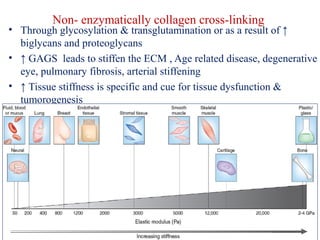
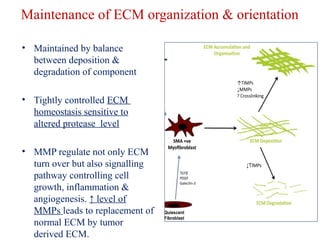
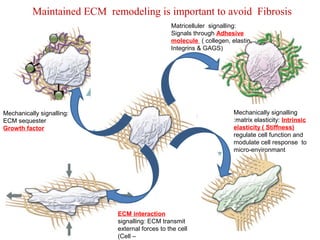
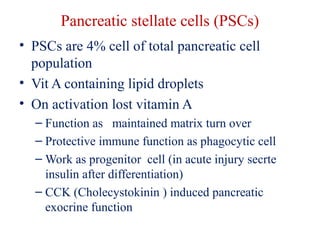
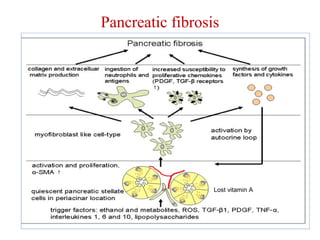
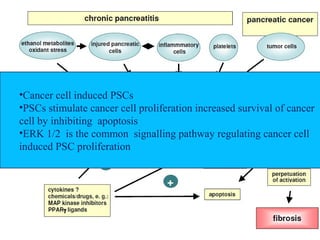
The document summarizes tissue regeneration and fibrosis. It covers the normal wound healing process and definitions of regeneration versus repair. It discusses stem cells, growth factors, signaling pathways, extracellular matrix components and cell-matrix interactions that are important for regeneration. Fibrosis occurs when there is excessive or uncontrolled deposition of extracellular matrix proteins like collagen. Maintaining the proper balance between deposition and degradation of extracellular matrix components is key to avoiding fibrosis and allowing normal tissue regeneration.