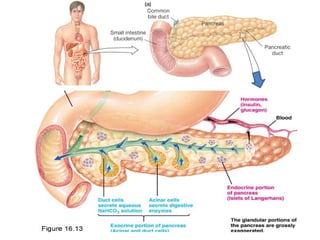
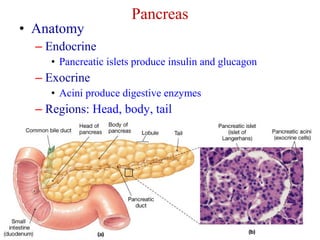
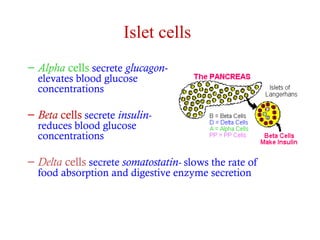
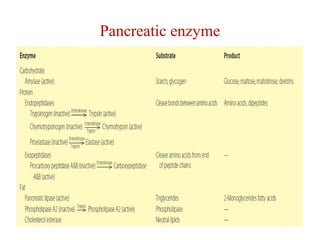
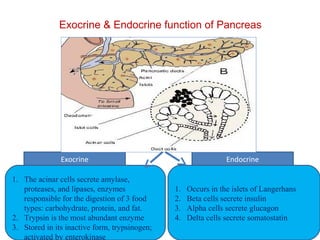
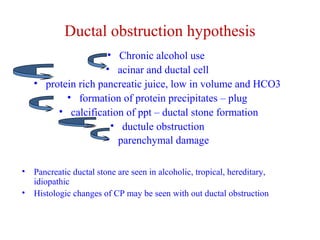
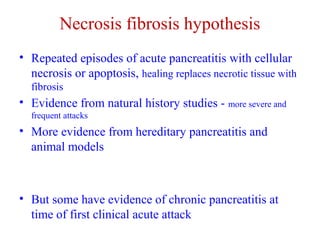
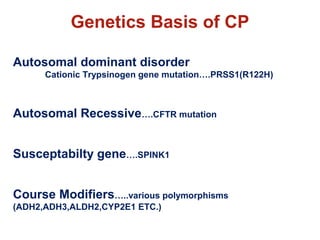
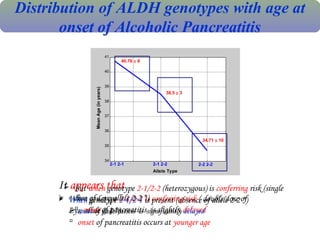
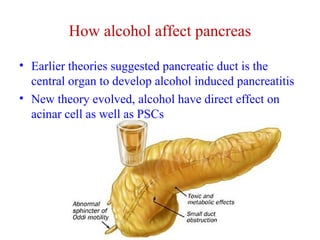
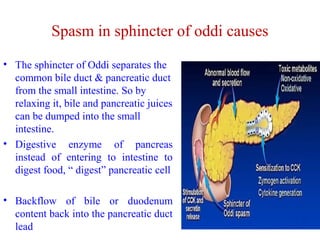
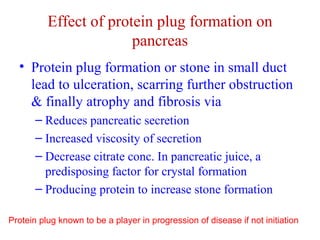
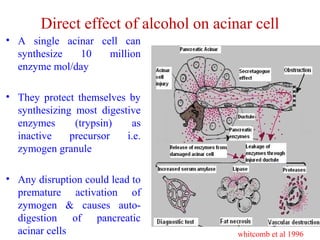
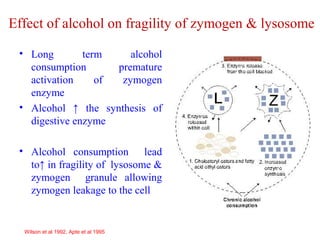
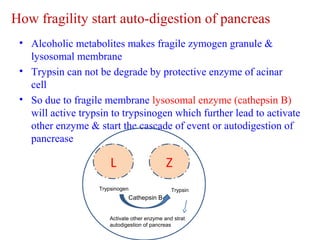
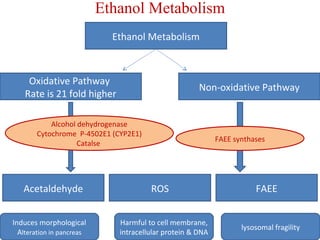
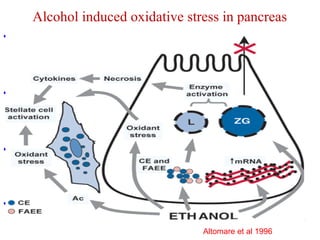
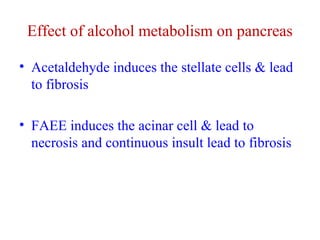
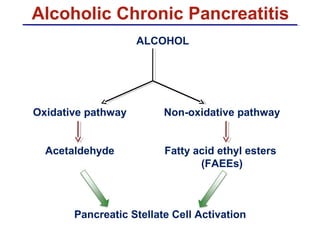
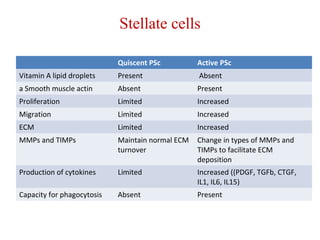
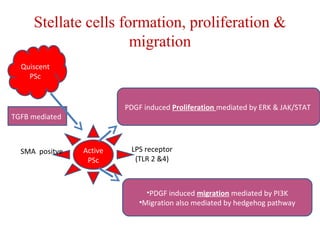
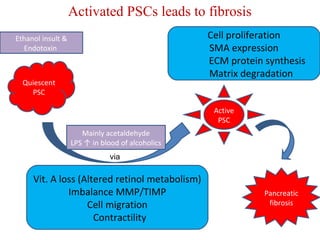
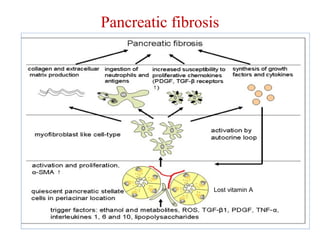
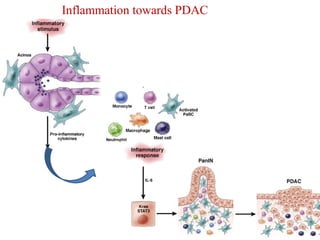
The document summarizes the effects of alcohol on the pancreas. It discusses how alcohol consumption can lead to pancreatic inflammation and fibrosis through several mechanisms. Chronic alcohol use can cause ductal obstruction from protein precipitates, increase the fragility of zymogen granules and lysosomes in acinar cells leading to premature enzyme activation and autodigestion, and induce oxidative stress. Alcohol metabolites also activate pancreatic stellate cells, which promote fibrosis through extracellular matrix deposition. Animal studies demonstrate models of pancreatic fibrosis induced by various insults like alcohol administration combined with caerulein injections or endotoxin exposure.