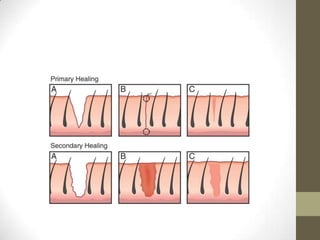
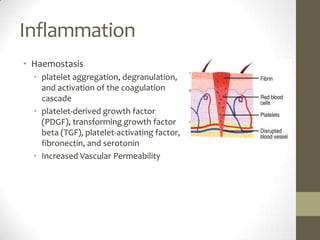
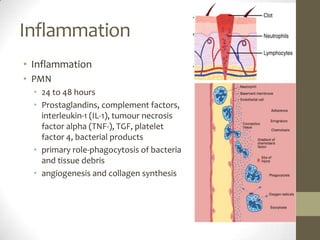
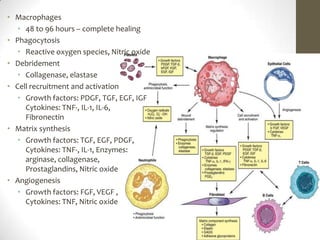
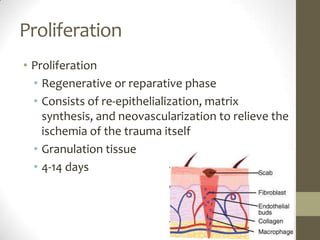
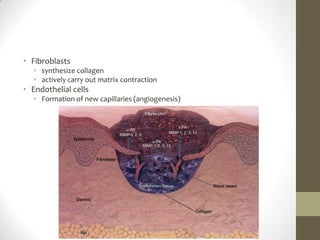
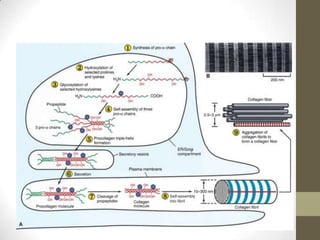
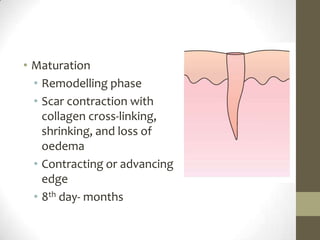
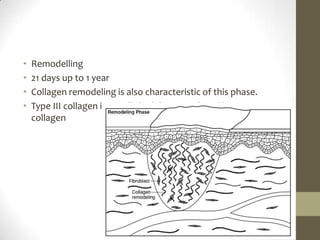
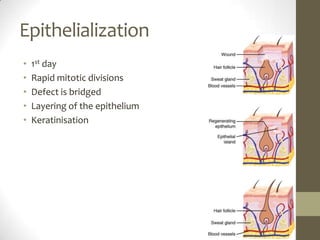
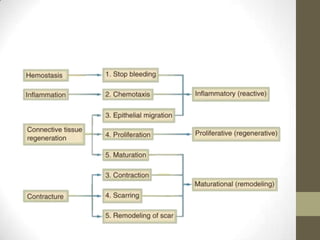
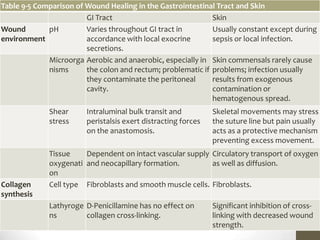
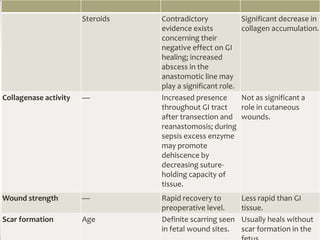
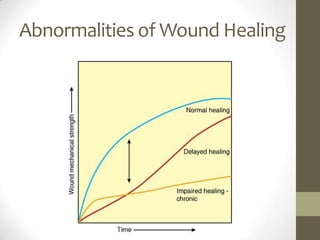
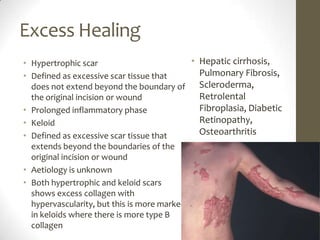
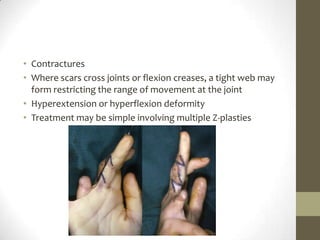
Wound healing involves three main phases - inflammation, proliferation, and maturation. During inflammation, hemostasis occurs within the first few hours followed by an inflammatory response involving neutrophils and macrophages over the next few days. Proliferation begins around 4-14 days as new tissue is formed through granulation, re-epithelialization, angiogenesis and collagen deposition by fibroblasts. Maturation occurs over weeks to months as remodeling and scar contraction takes place. Abnormal wound healing can result in excessive scarring like hypertrophic scars and keloids. Treatment depends on the type of abnormality but may involve pressure, silicone sheeting, steroid injections, excision and radiation. Contractures can also form from scars limiting