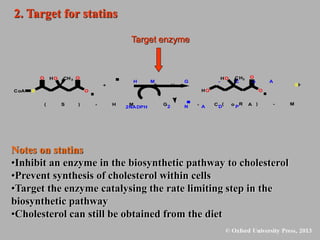
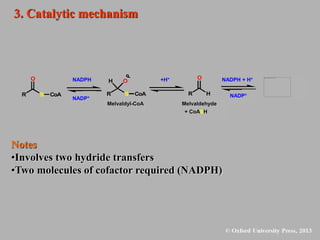
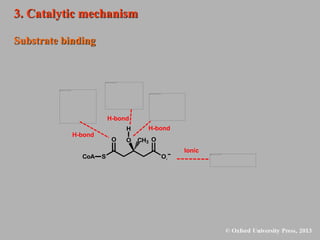
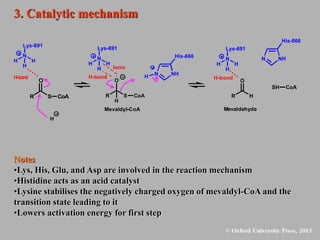
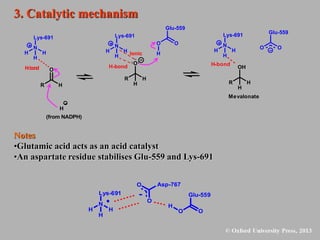
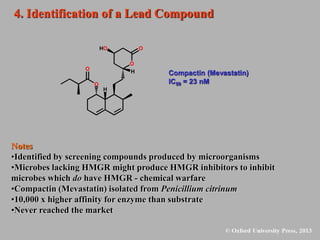
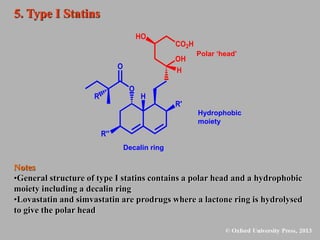
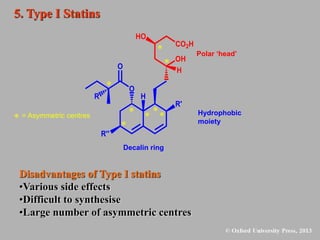
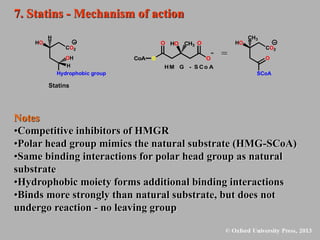
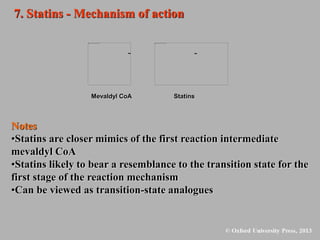
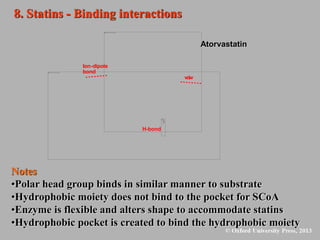
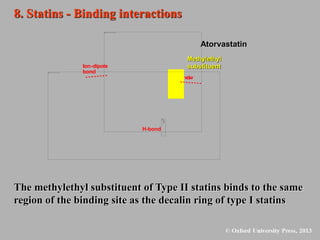
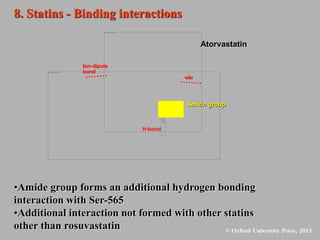
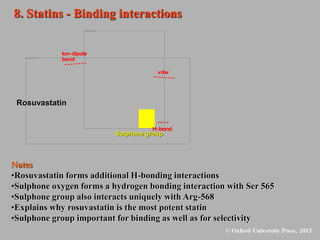
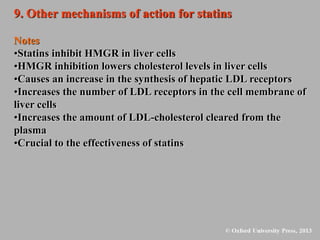
This document discusses statins, which are cholesterol-lowering drugs. It notes that excess cholesterol can cause cardiovascular issues. Statins work by inhibiting HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. The first identified statin was mevastatin from a fungus. Later statins included lovastatin, simvastatin, and pravastatin. Newer "type II" statins like atorvastatin and rosuvastatin are easier to synthesize. Statins bind competitively to HMG-CoA reductase, forming additional interactions beyond the natural substrate. Rosuvastatin forms unique bonds and is the most potent statin. Statins' main mechanism is reducing