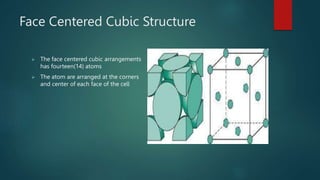
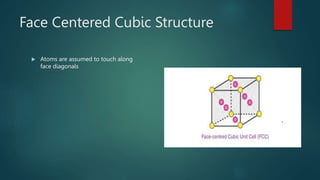
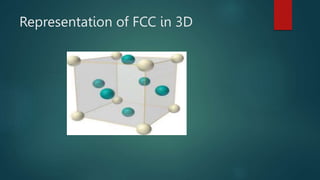
The document discusses the face centered cubic (FCC) crystal structure. It describes how in an FCC structure, atoms are arranged at the corners and center of each face of the cubic unit cell, totaling 14 atoms per cell. Atoms in FCC have a coordination number of 12 and high atomic packing factor due to dense packing. Common metals that adopt the FCC structure include aluminum, copper, nickel, iron, gold and silver.