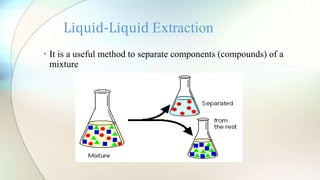
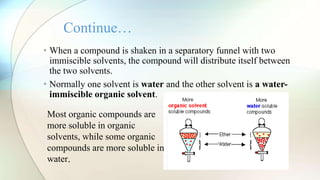
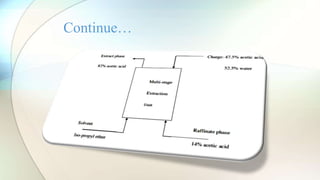
The document discusses liquid-liquid extraction, a process used to separate components from mixtures that cannot be easily separated by distillation. It explains the formation of immiscible layers when a solvent is added, along with examples illustrating the principles of extraction and efficiency enhancement techniques. Additionally, a calculation example is provided to determine the percentage of acetic acid remaining unextracted from a mixture.