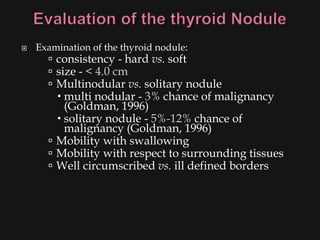
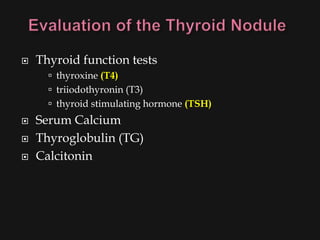
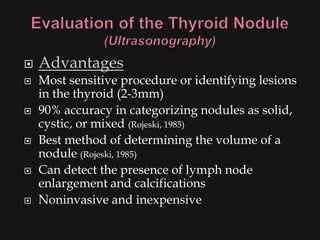
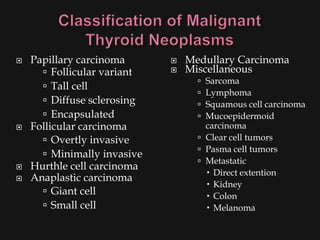
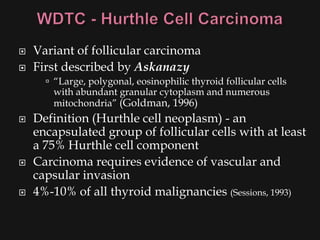
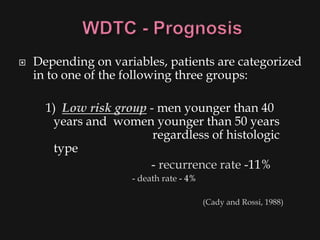
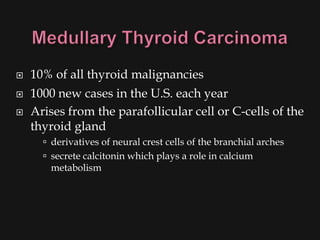
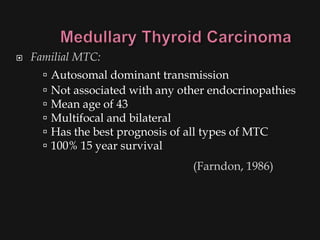
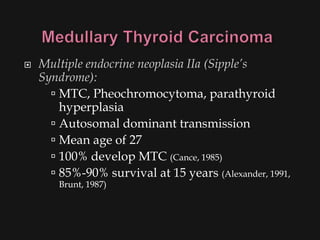
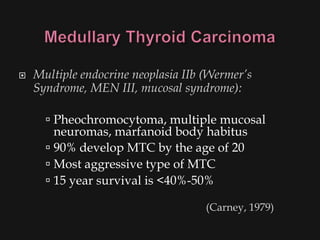
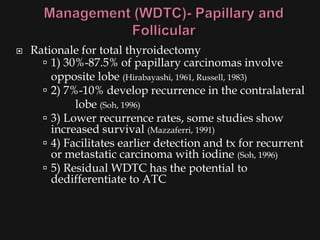
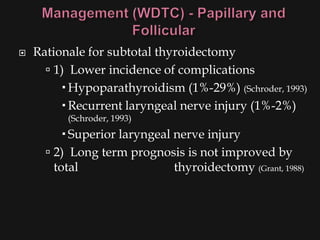
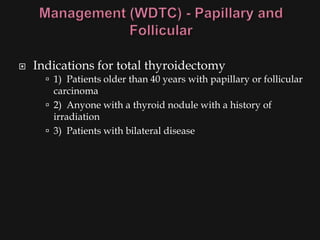
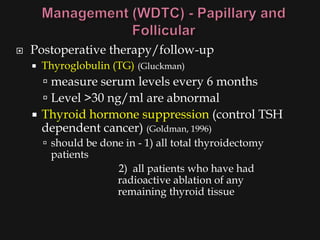
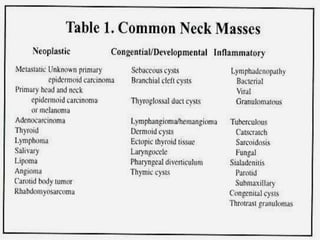
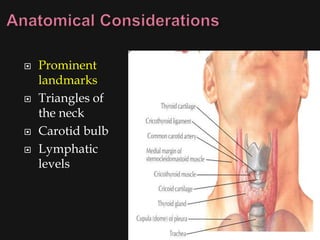
This document provides an overview of evaluating neck masses, with a focus on the thyroid gland. It discusses the anatomy of the neck, characteristics of common neck masses, diagnostic tools such as biopsy and imaging, and characteristics and management of various thyroid conditions. Prominent topics include thyroid cancer risk factors, symptoms of malignant thyroid nodules, and examination findings suggestive of thyroid cancer.
























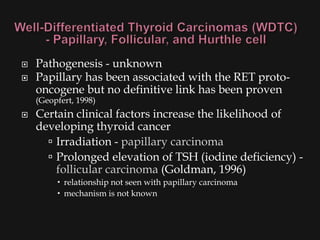
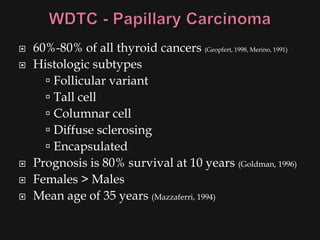
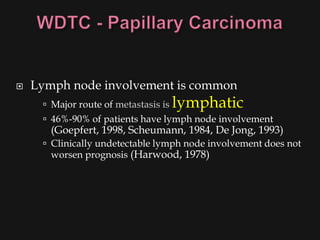
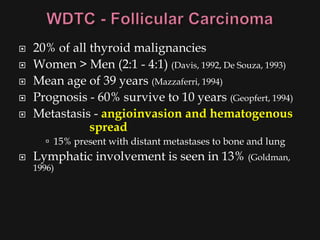
![ Risk factors
Thyroid exposure to irradiation
low or high dose external irradiation (40-50 Gy
[4000-5000 rad])
especially in childhood for:
large thymus, acne, enlarged tonsils, cervical
adenitis, sinusitis, and malignancies
30%-50% chance of a thyroid nodule to be
malignant (Goldman, 1996)
Schneider and co-workers (1986) studied, with
long term F/U, 3000 patients who underwent
childhood irradiation.
1145 had thyroid nodules
318/1145 had thyroid cancer (mostly papillary)](https://image.slidesharecdn.com/evaluationoftheneck-120306180403-phpapp02/85/Evaluation-of-the-neck-44-320.jpg)