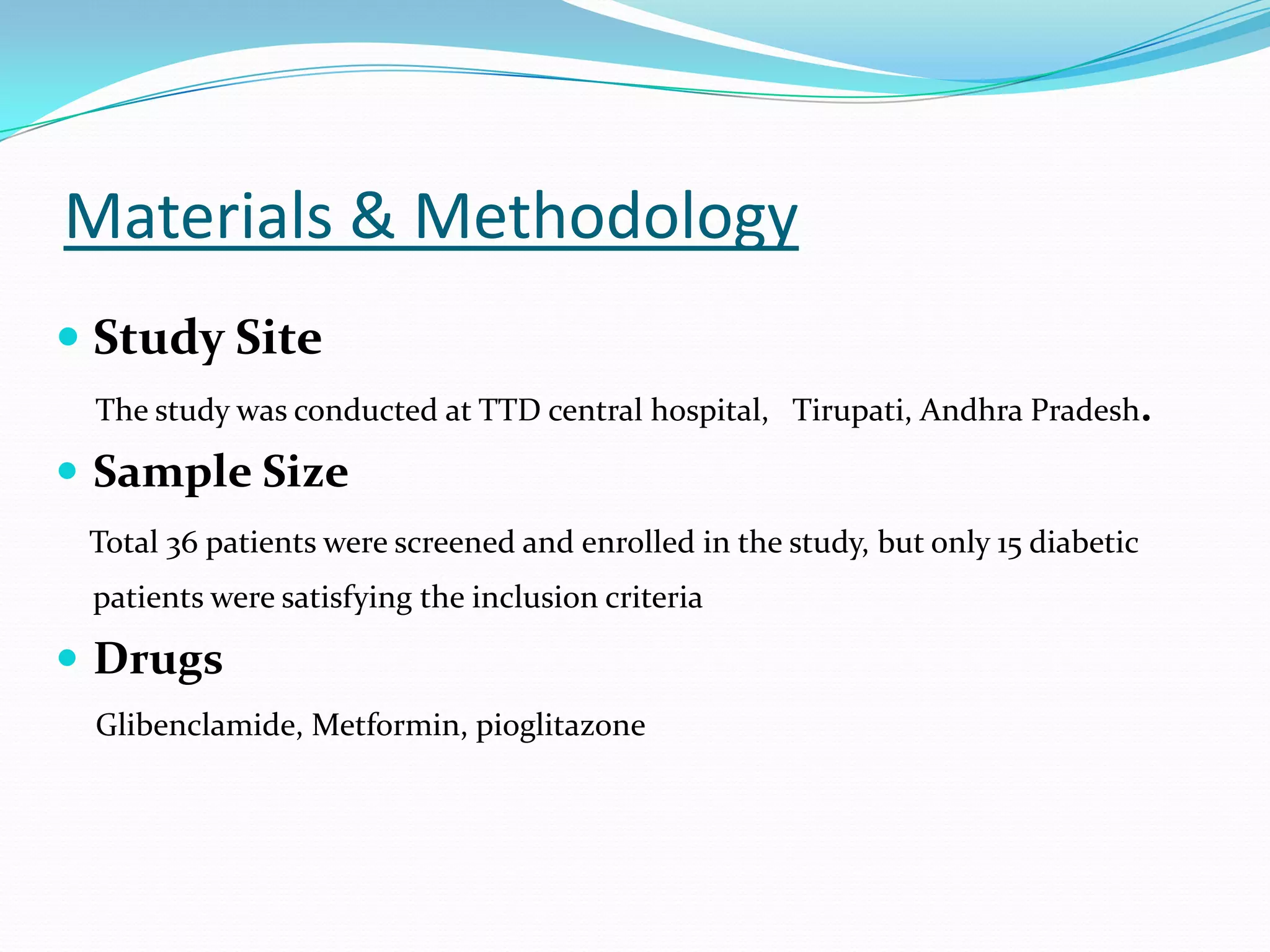
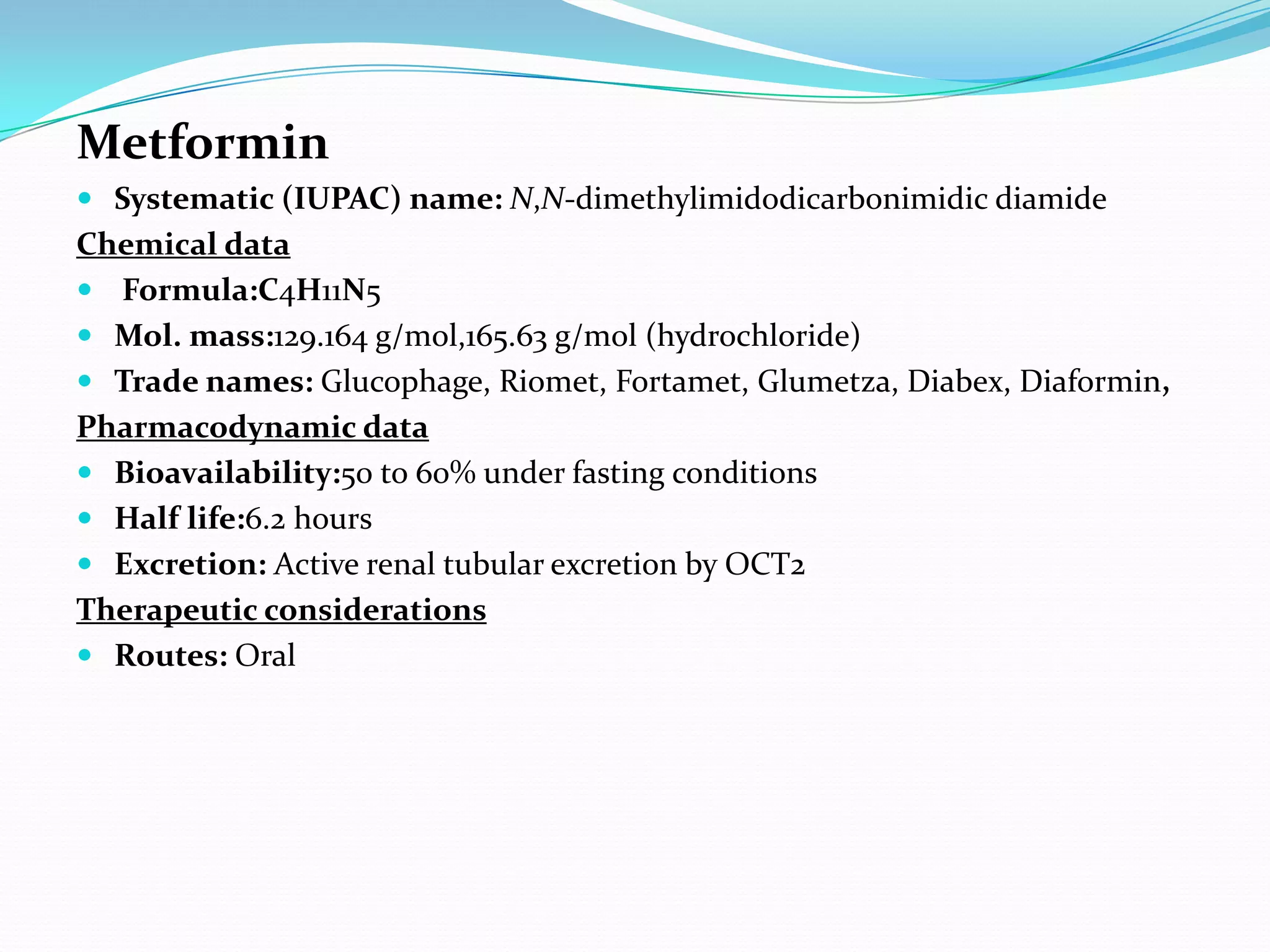
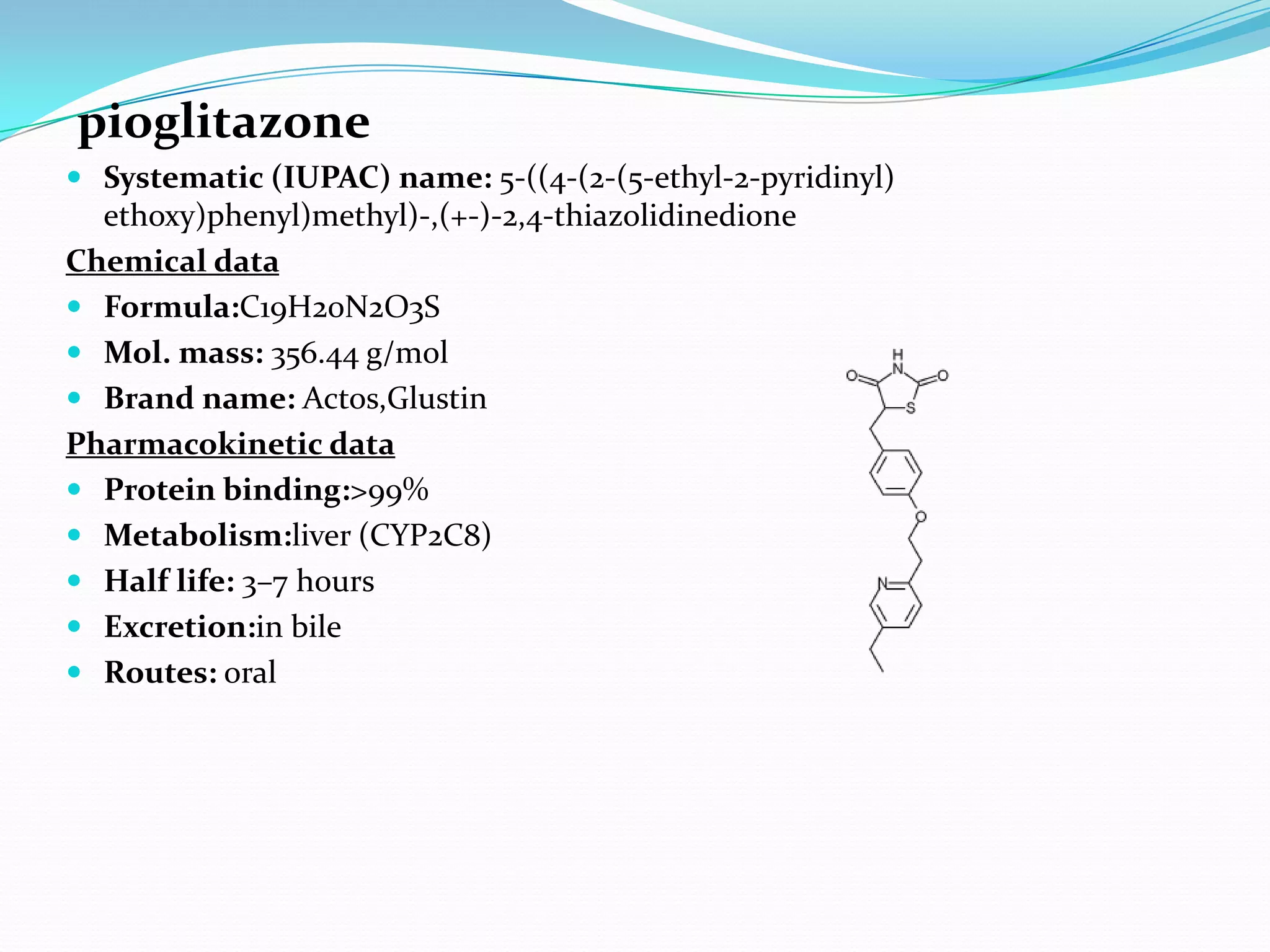
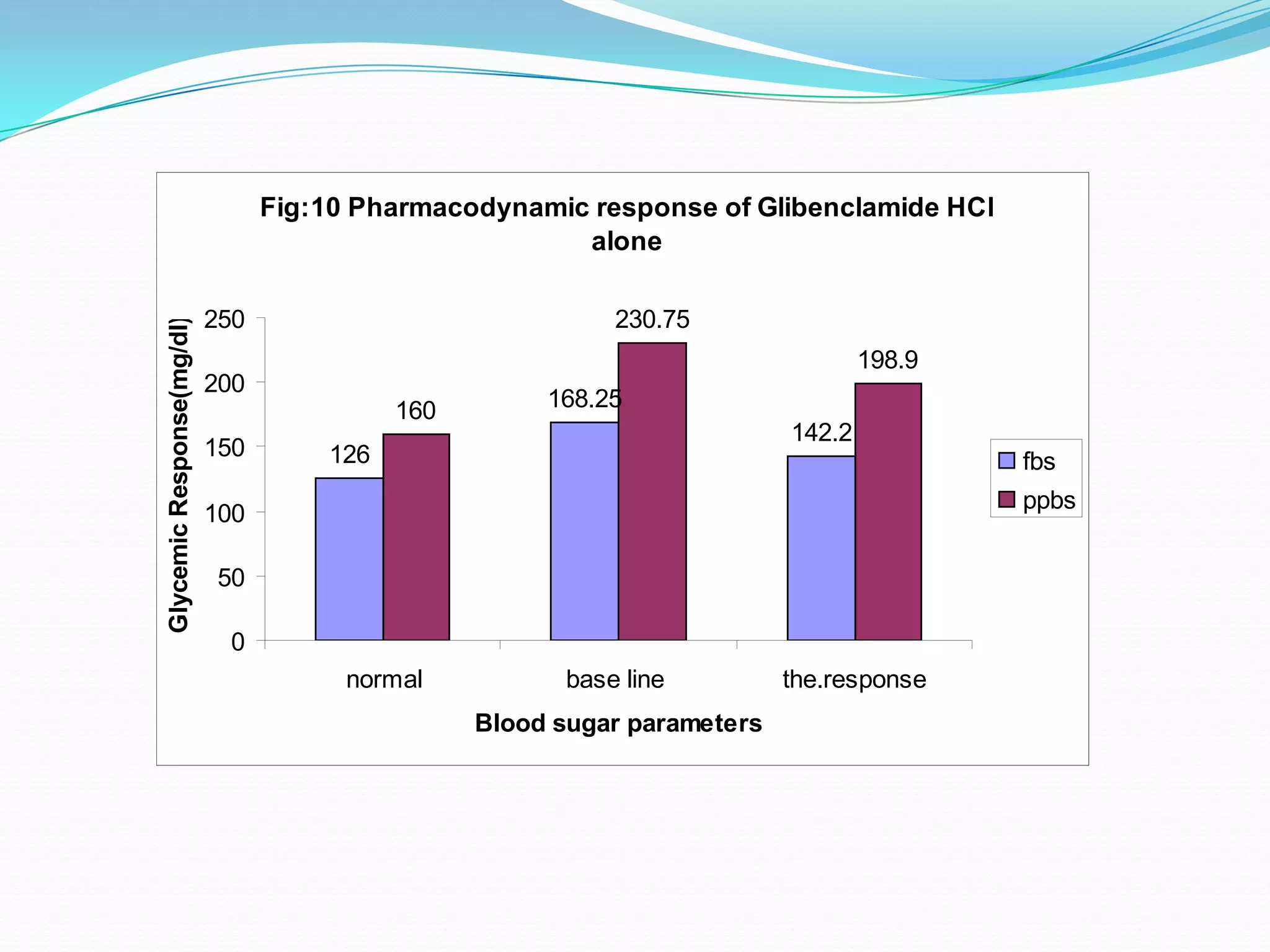
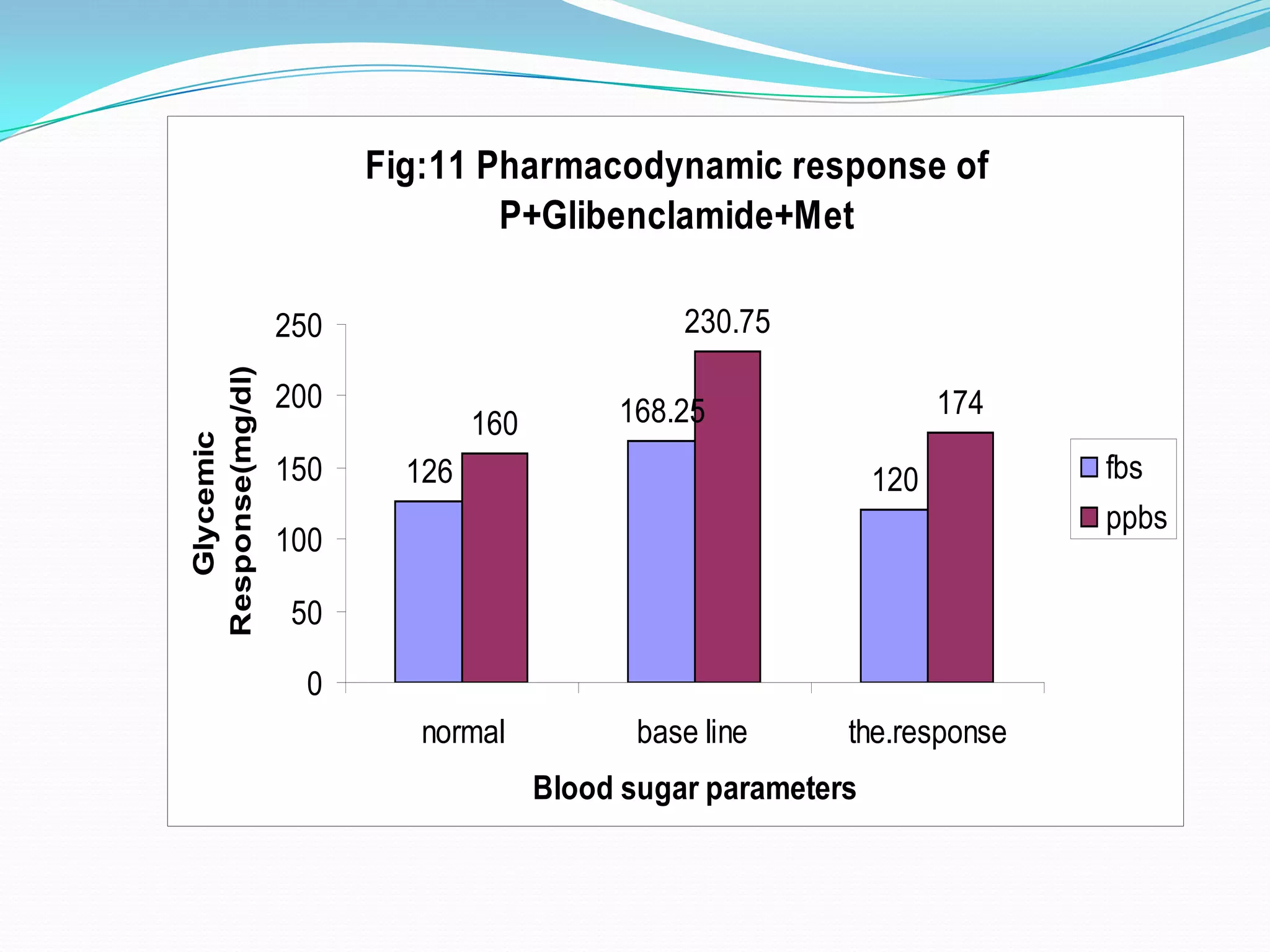
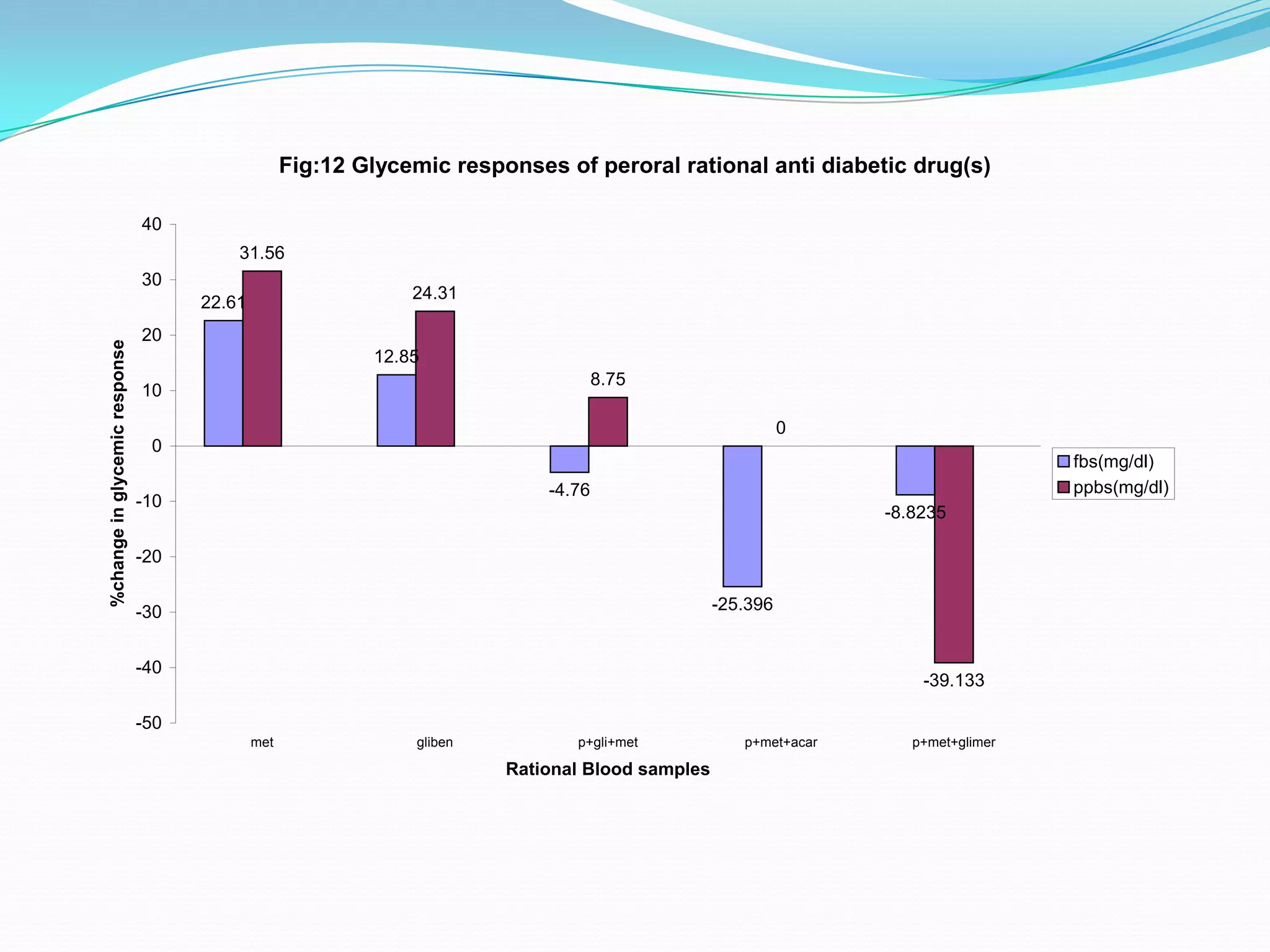
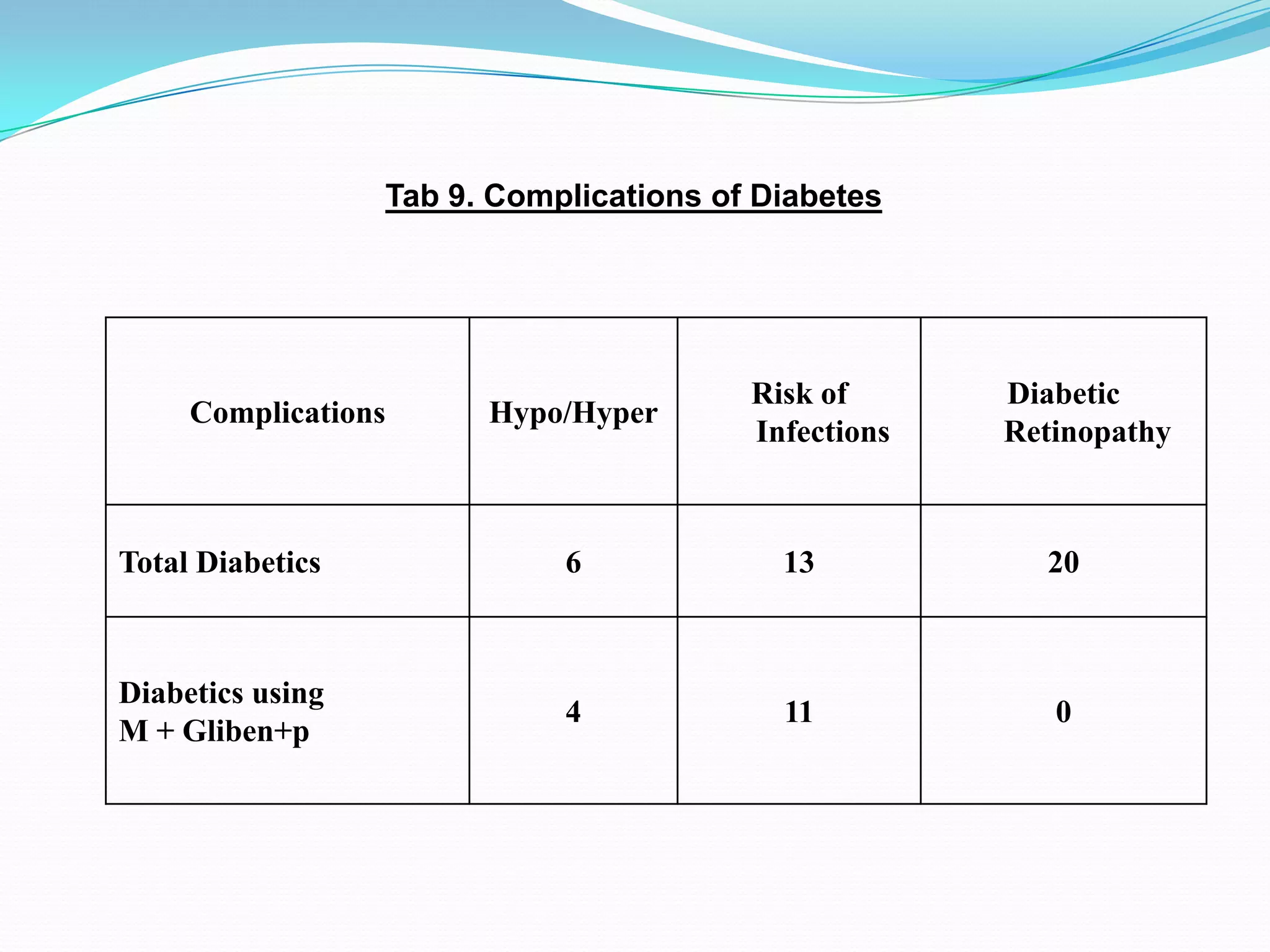
This dissertation evaluates the glycemic response of adding pioglitazone to glibenclamide and metformin in type 2 diabetic patients compared to monotherapy with glibenclamide and metformin. The study involved a total of 36 screened patients, with 15 meeting inclusion criteria, and assessed the efficacy, adverse reactions, and complications associated with the treatment regimens over six months. The results indicated that the combination therapy provided superior glycemic control and improved tolerability at lower doses, suggesting its potential to postpone insulin therapy.








![Drug profiles
Glibenclamide
Systematic (IUPAC) name: 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl) phenyl]ethyl]-2-
methoxy-benzamide
Chemical data
Formula: C23H28ClN3O5S
Mol. mass: 494.004 g/mol
Pharmacokinetic data
Protein binding: Extensive
Metabolism: Hepatic hydroxylation (CYP2C9-mediated)
Half life: 10 hours
Excretion: Renal and biliary
Therapeutic considerations
Routes :Oral
Dose: 1.25mg,2.5mg,and 5mg
Trade names: Daonil, Euglucon.
Mechanism of action:“ inhibiting ATP-sensitive potassium channels in pancreatic beta cells”.](https://image.slidesharecdn.com/d-170322140537/75/EVALUATION-OF-GLYCEMIC-RESPONSE-OF-ADDITION-OF-PIOGLITAZONE-TO-GLIBENCLAMIDE-AND-METFORMIN-HCL-IN-TYPE-2-DIABETIC-PATIENTS-ON-COMPARISON-WITH-GLIBENCLAMIDE-AND-METFORMIN-MONOTHERAPY-10-2048.jpg)

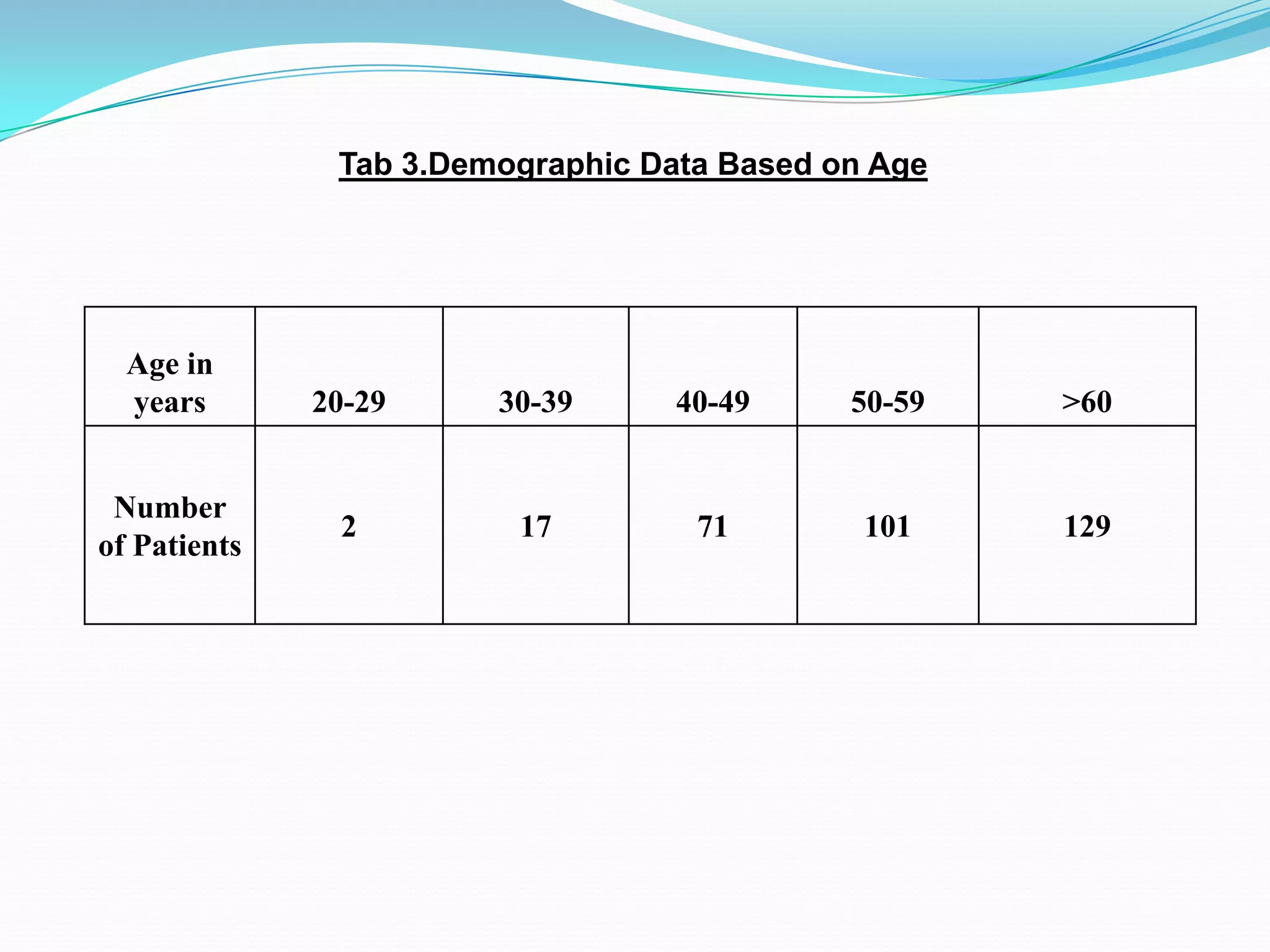
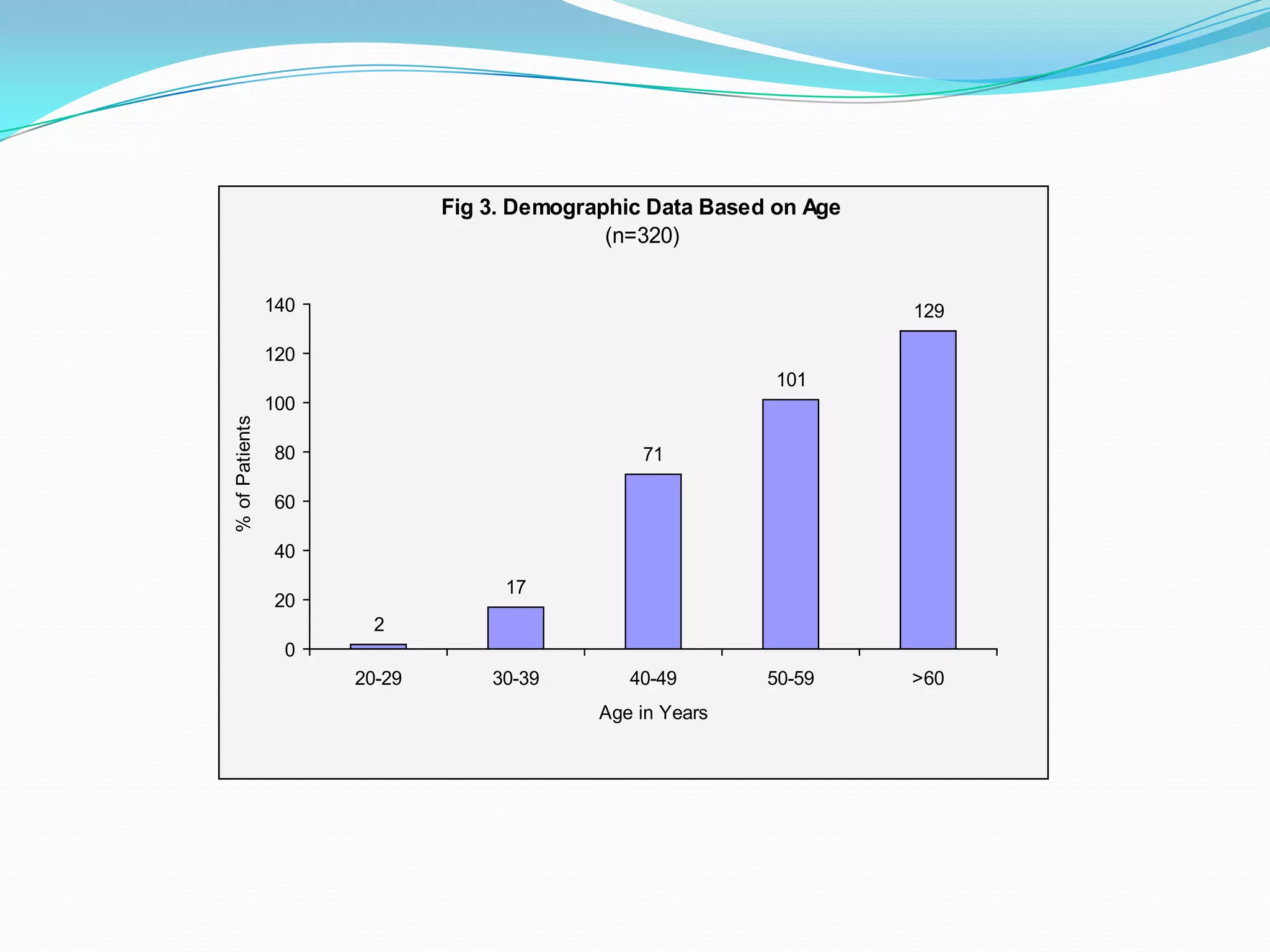
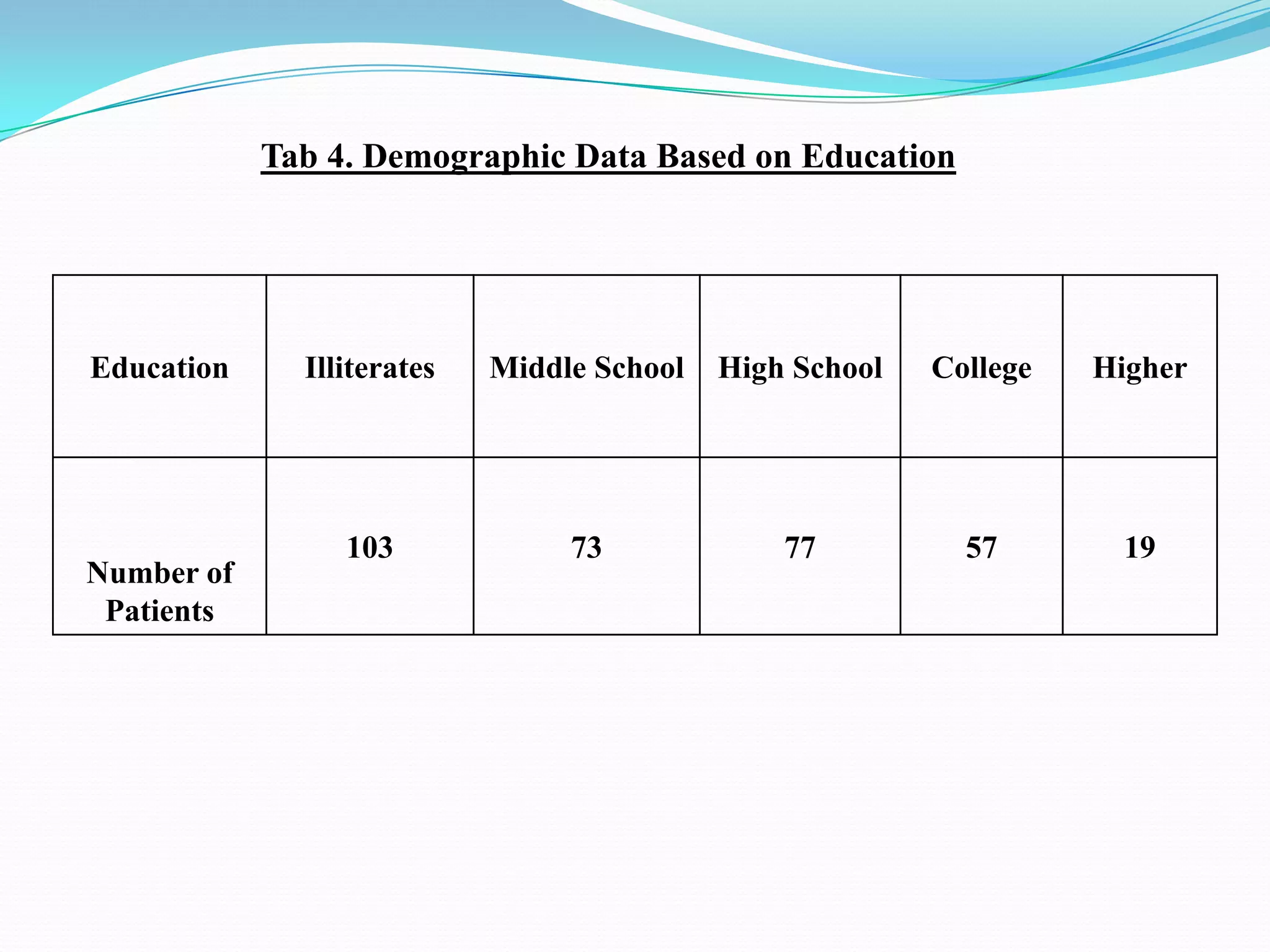
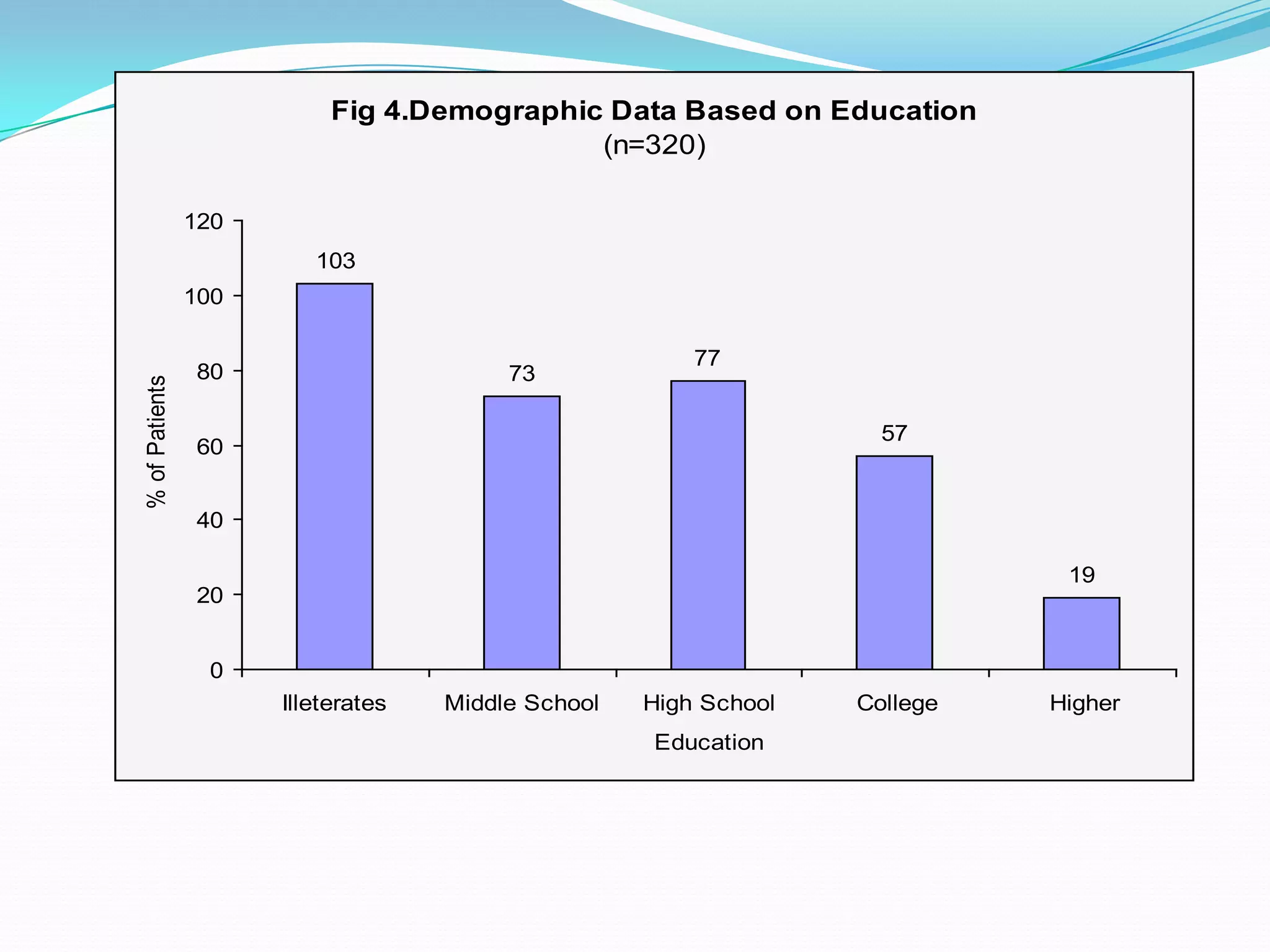
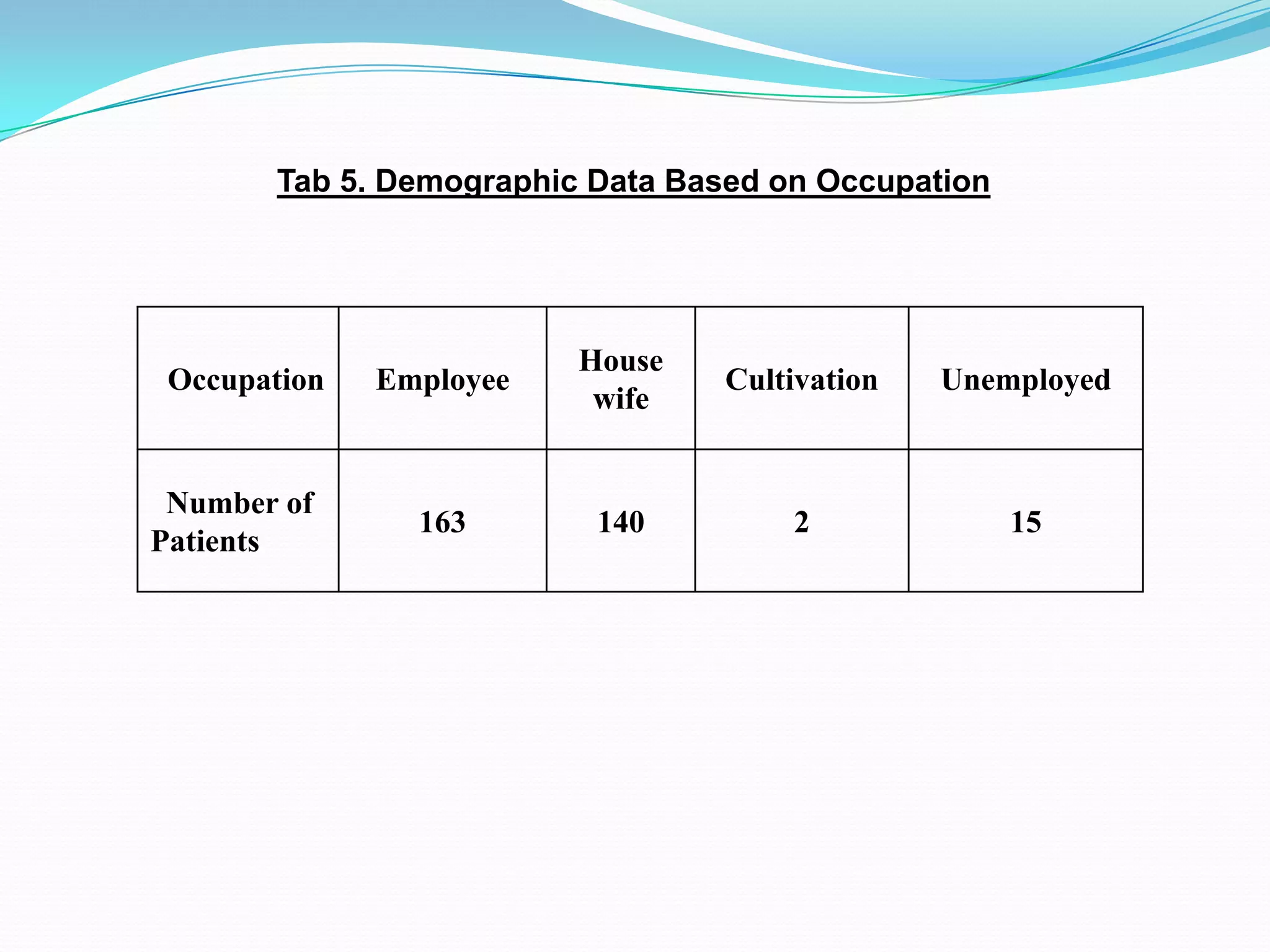
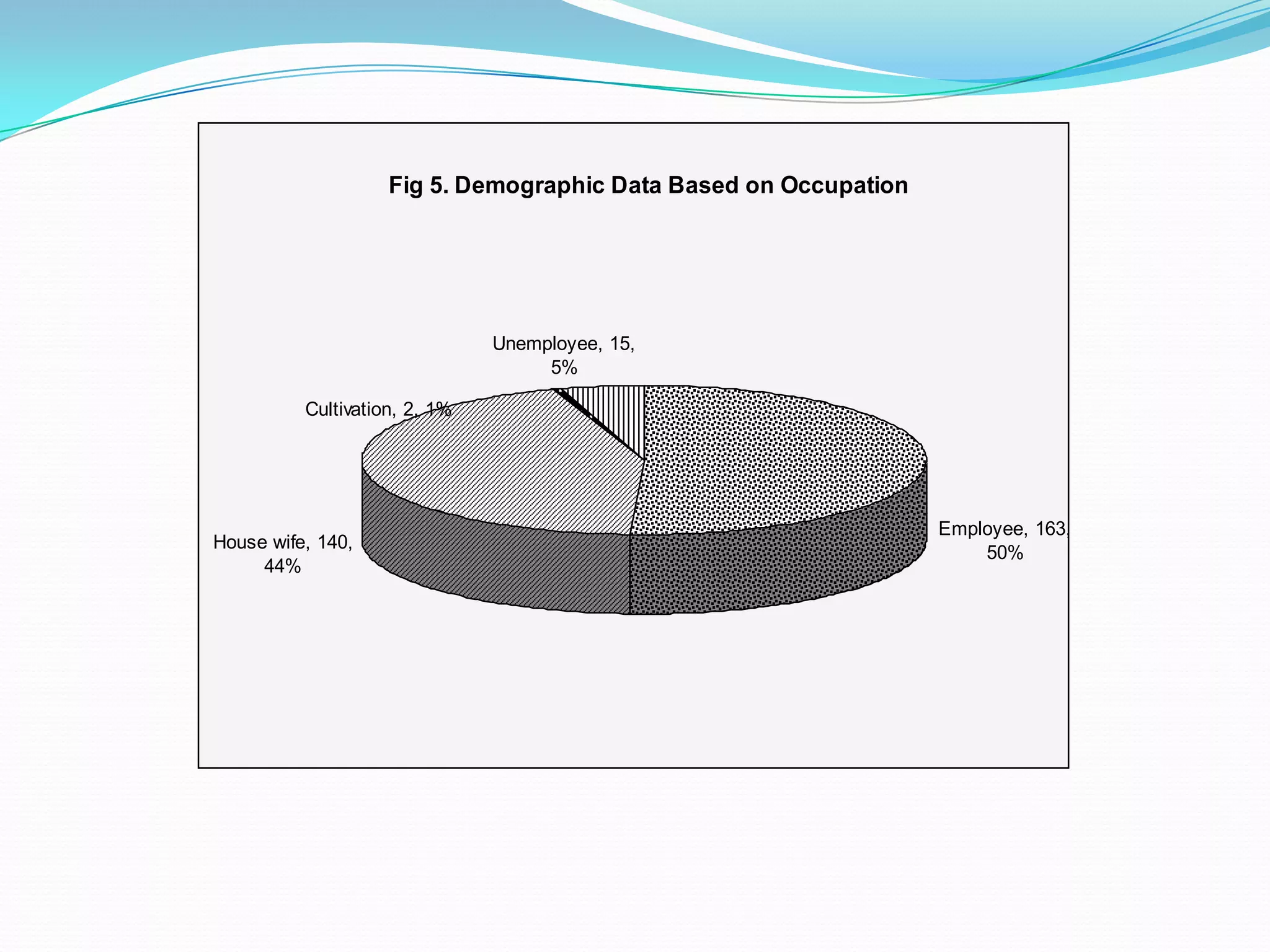
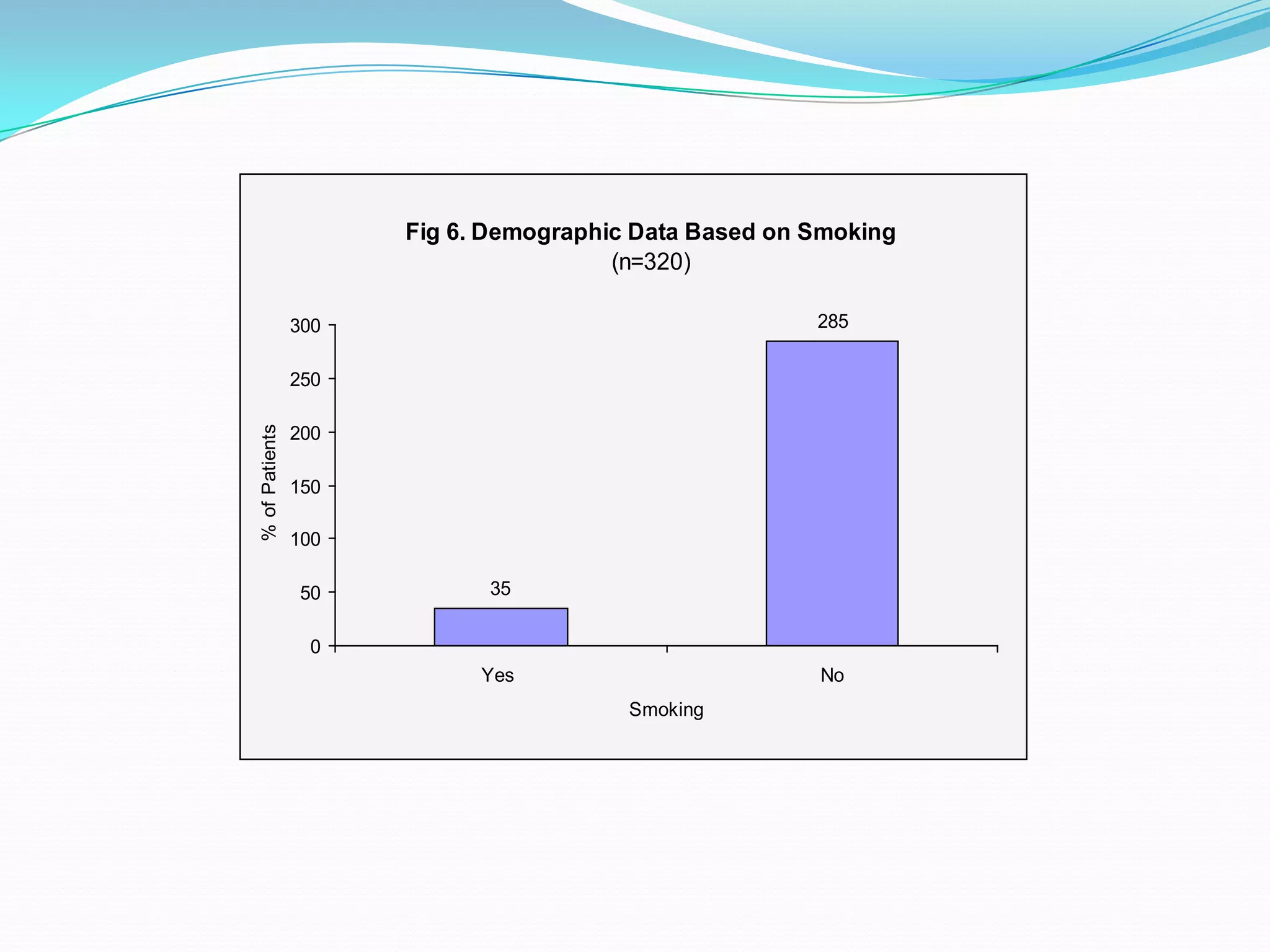
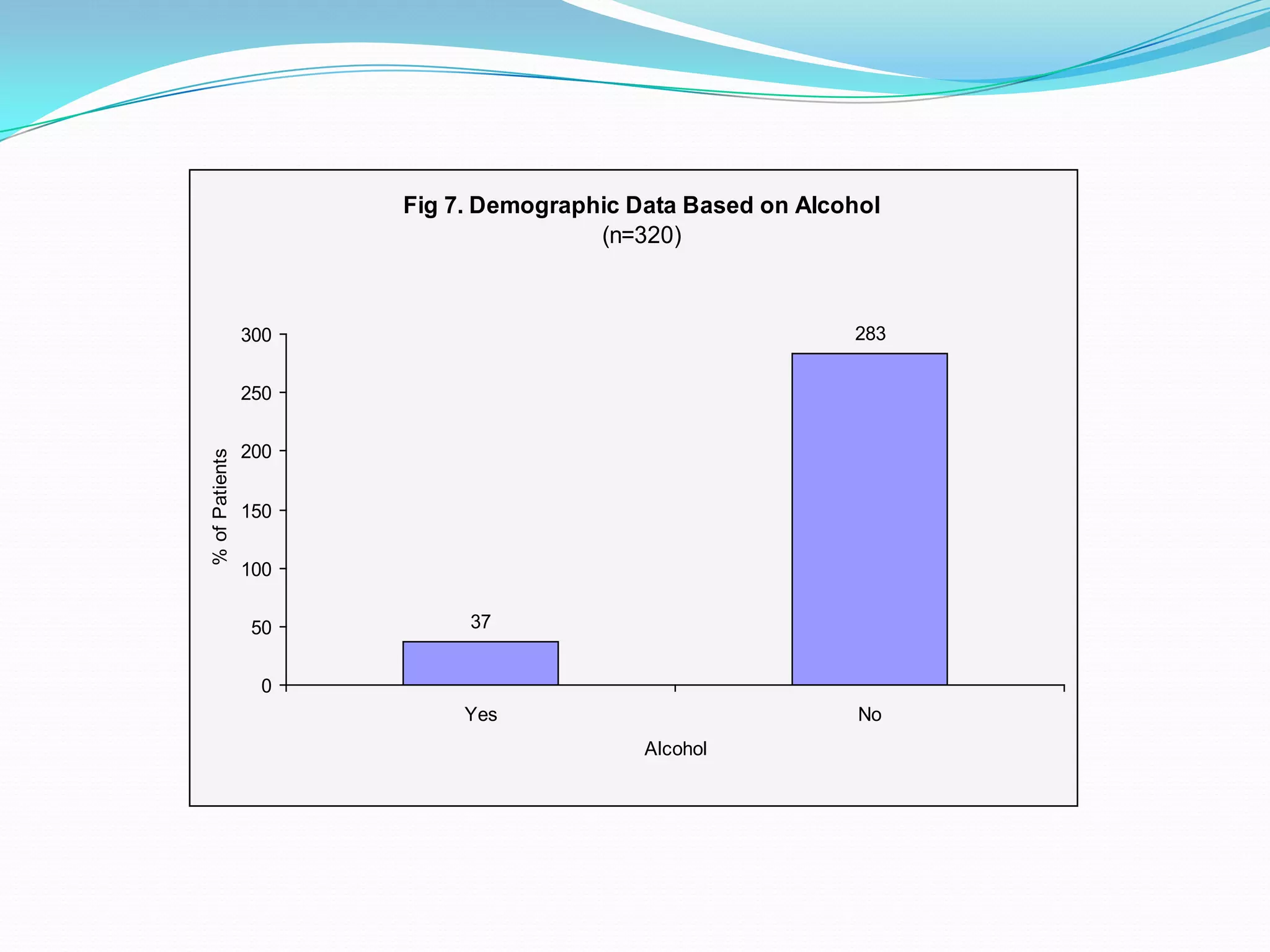

![References
IDF Chooses Blue Circle to Represent UN Resolution Campaign. Unite for Diabetes (17 March
2006).
L M Tierney, S J McPhee, M A Papadakis (2002). Current medical Diagnosis & Treatment.
International edition. New York: Lange Medical Books/McGraw-Hill, 1203-1215. ISBN 0-07137688-7.
World Health Organisation Department of Noncommunicable Disease Surveillance (1999).
Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications (PDF).
Rother, KI (2007). "Diabetes Treatment — Bridging the Divide". N Engl J Med 356 (15): 1499-1501.
Mailloux, Lionel (2007-02-13). Template:Cite web
url=http://patients.uptodate.com/topic.asp?file=dialysis/15147/title=UpToDate Dialysis in
diabetic nephropathy /accessdate=2007-12-07 /format= /work=]] UpToDate Dialysis in diabetic
nephropathy]. UpToDate. Retrieved on 2007-12-07.
Other "types" of diabetes. American Diabetes Association (August 25, 2005).
Diseases: Johns Hopkins Autoimmune Disease Research Center. Retrieved on 2007-09-23.
FDA Approves First Ever Inhaled Insulin Combination Product for Treatment of Diabetes.
Retrieved on 2007-09-09.
Eberhart, MS; Ogden C, Engelgau M, Cadwell B, Hedley AA, Saydah SH (November 19, 2004).
"Prevalence of Overweight and Obesity Among Adults with Diagnosed Diabetes ---United States,
1988--1994 and 1999--2002". Morbidity and Mortality Weekly Report 53 (45): 1066-1068. Centers for
Disease Control and Prevention. Retrieved on 2007-03-11](https://image.slidesharecdn.com/d-170322140537/75/EVALUATION-OF-GLYCEMIC-RESPONSE-OF-ADDITION-OF-PIOGLITAZONE-TO-GLIBENCLAMIDE-AND-METFORMIN-HCL-IN-TYPE-2-DIABETIC-PATIENTS-ON-COMPARISON-WITH-GLIBENCLAMIDE-AND-METFORMIN-MONOTHERAPY-39-2048.jpg)





