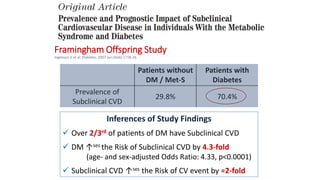
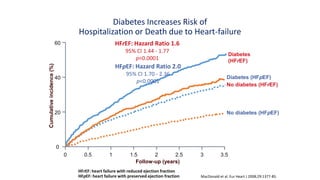
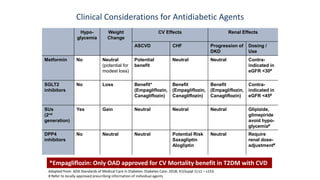
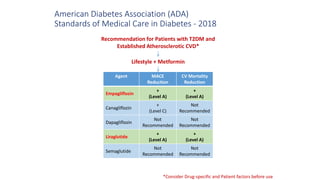
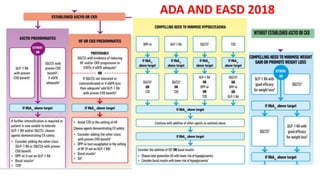
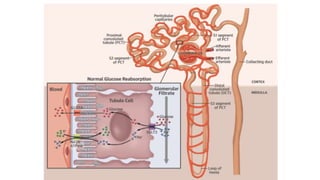
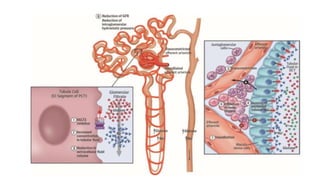
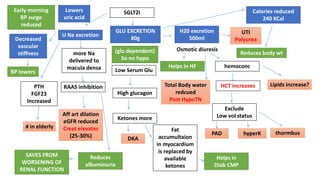
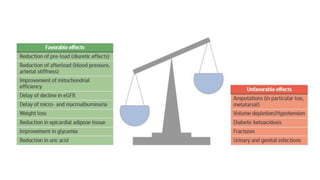
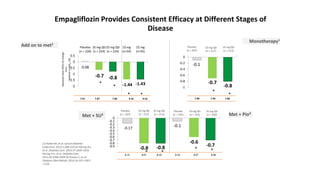
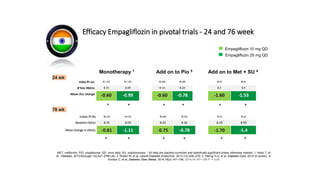
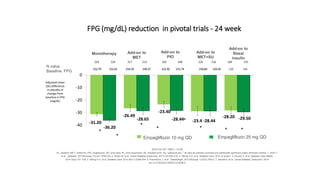
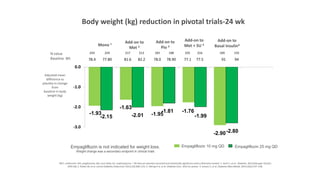
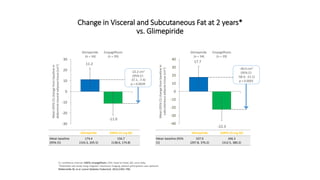
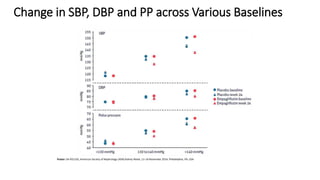
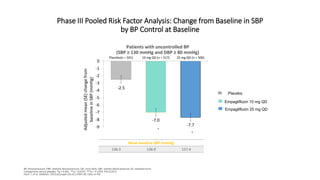
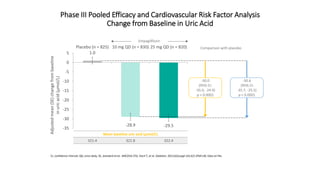
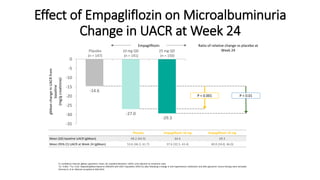
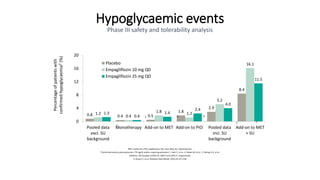
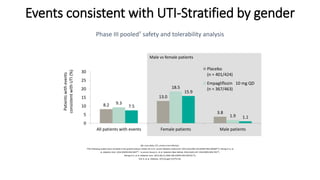
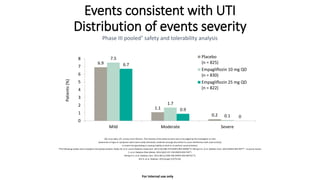
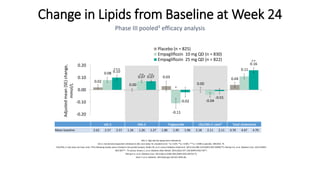
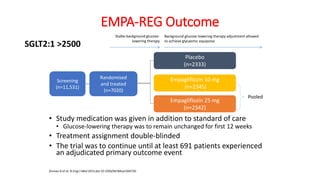
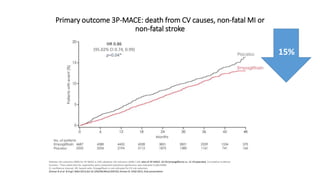
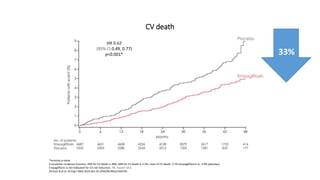
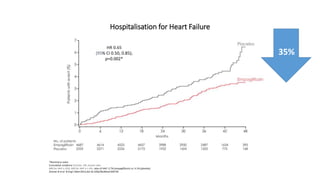
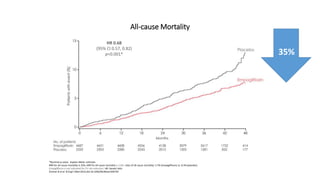
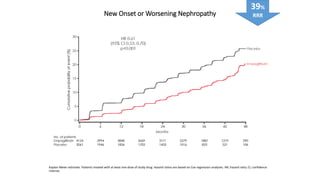
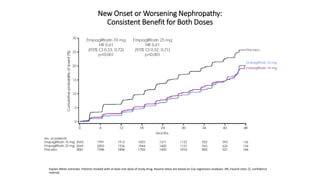
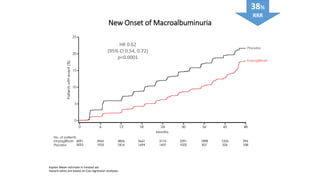
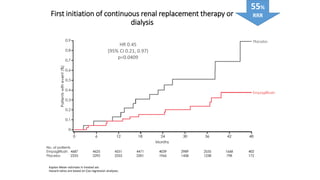
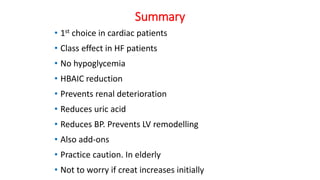
This document discusses the use of SGLT2 inhibitors (SGLT2i) in managing diabetes. It presents three case studies of patients with diabetes and cardiovascular complications who may benefit from SGLT2i treatment. It summarizes clinical trial data showing that empagliflozin lowers HbA1c, fasting plasma glucose, body weight, and blood pressure compared to other antidiabetic drugs. Empagliflozin also reduces visceral and subcutaneous fat. The document concludes that SGLT2i like empagliflozin provide glycemic control and cardiovascular benefits and can be considered as an addition to metformin for treating diabetes.