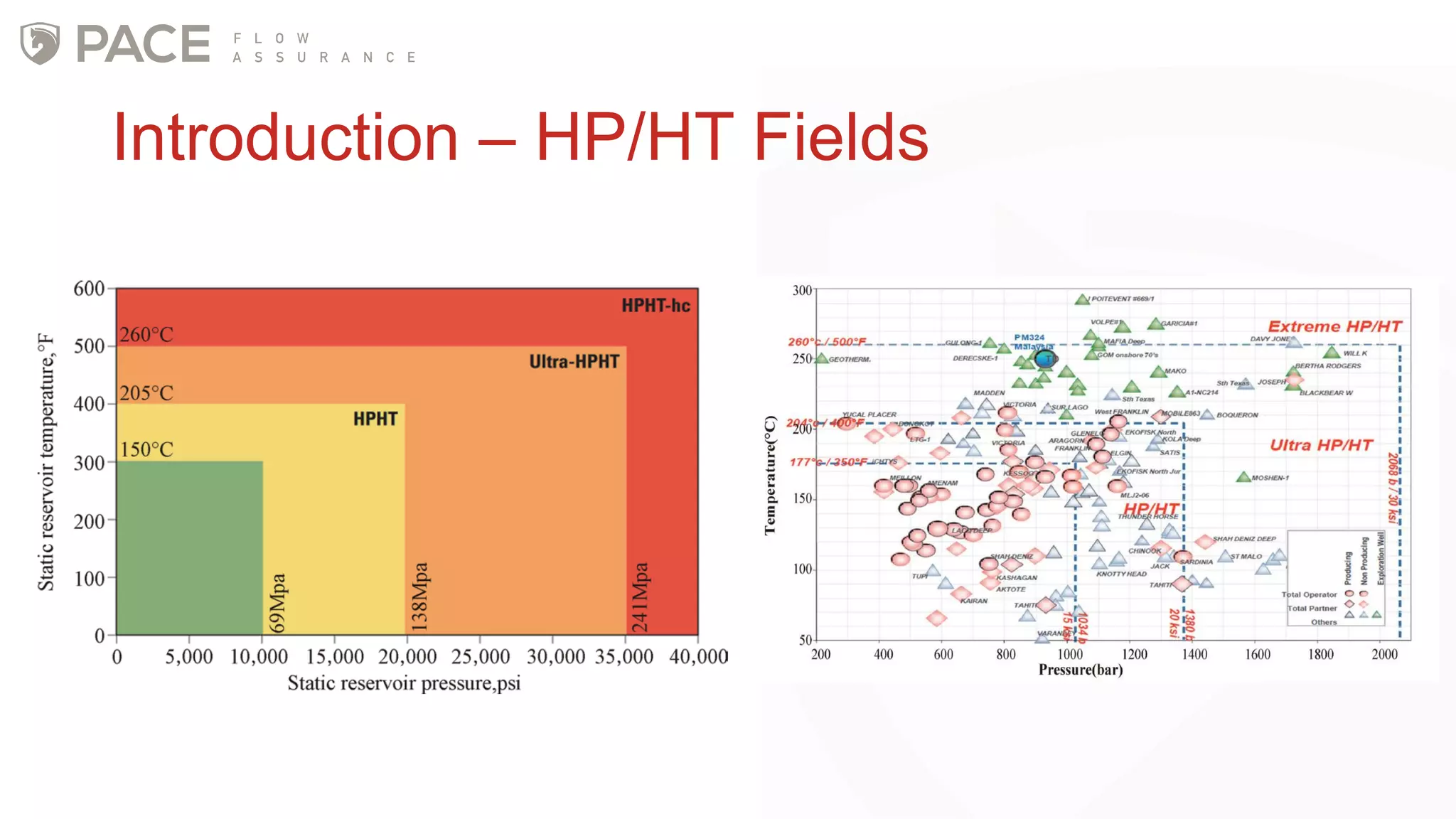
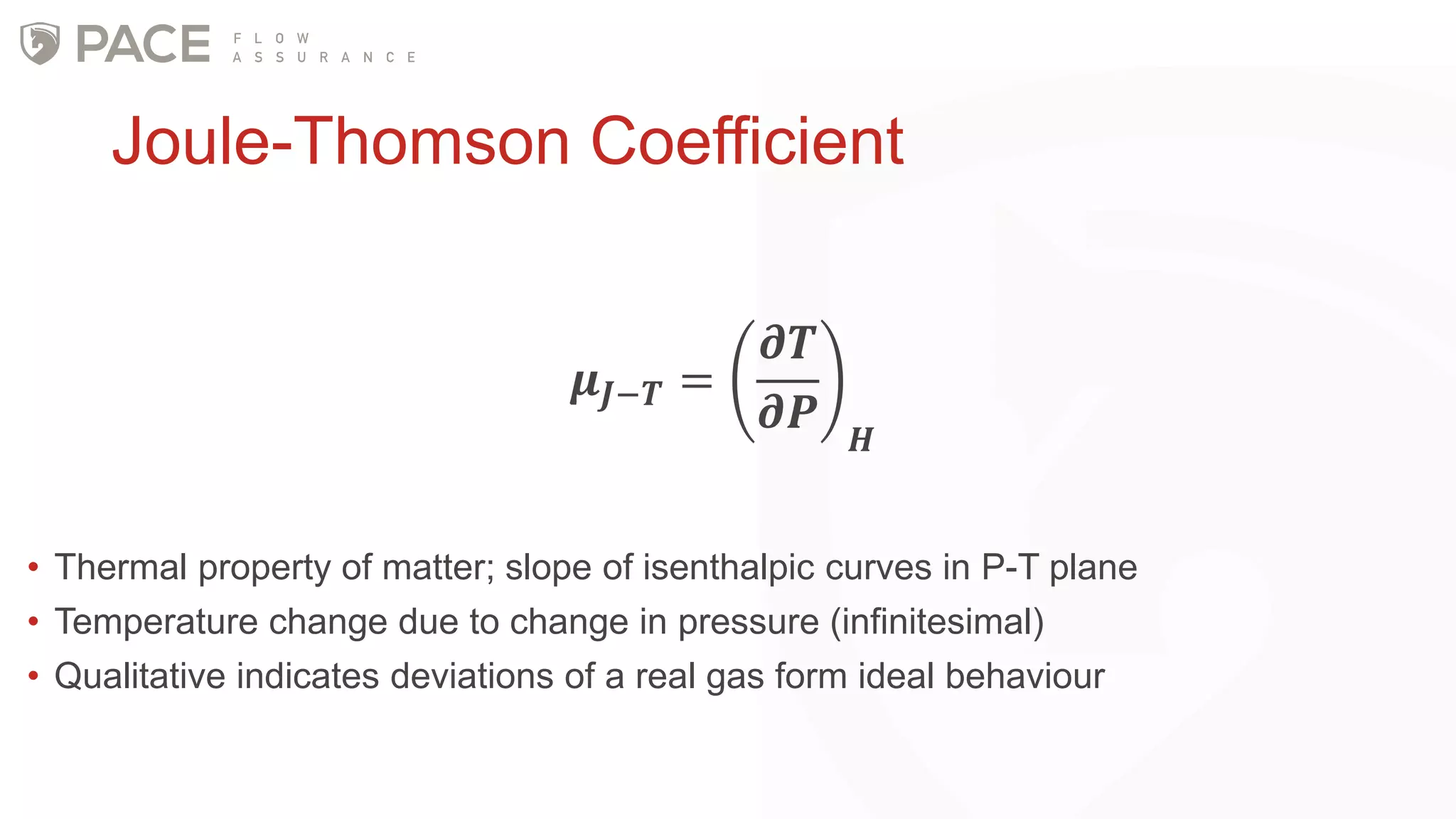
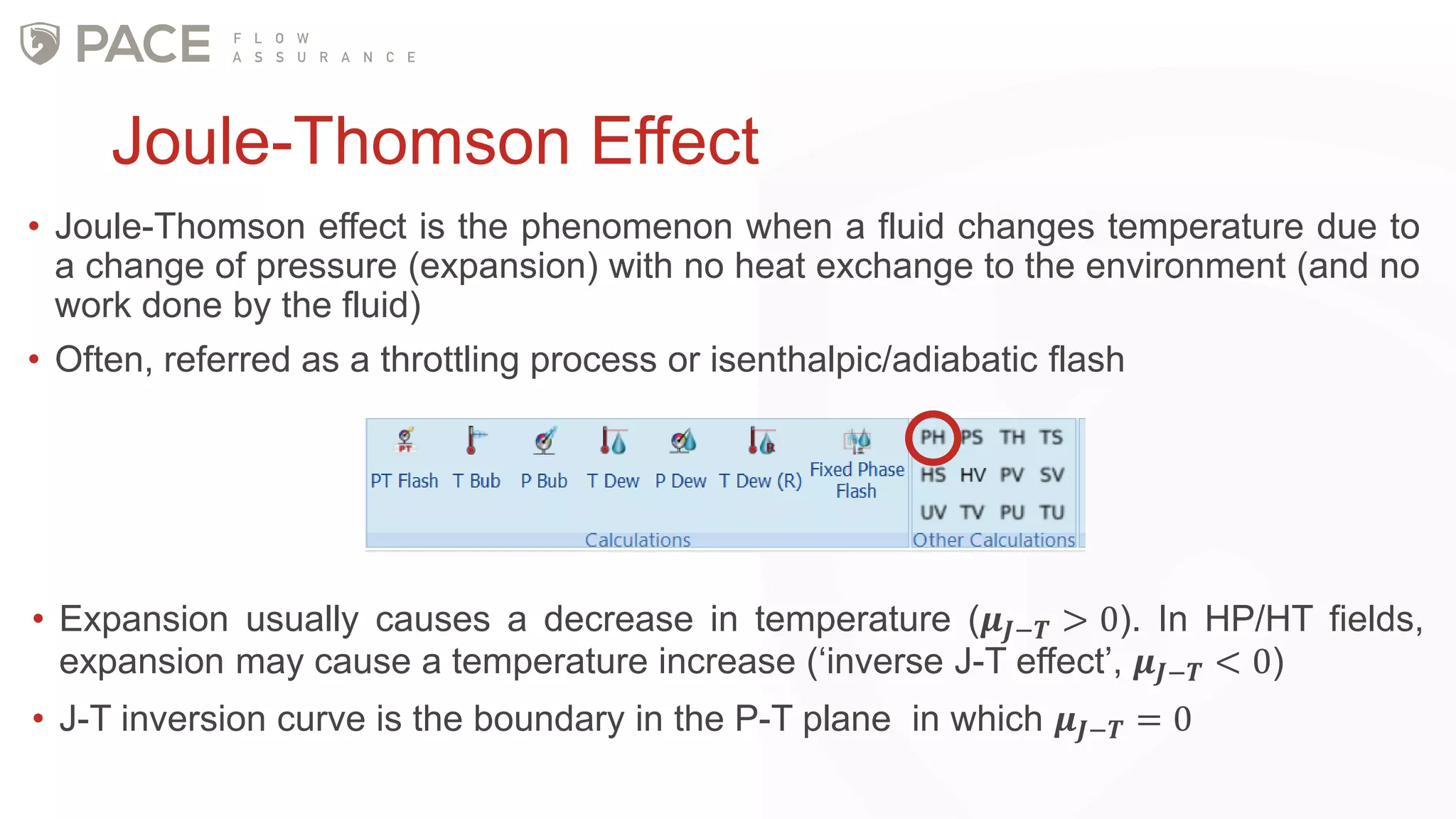
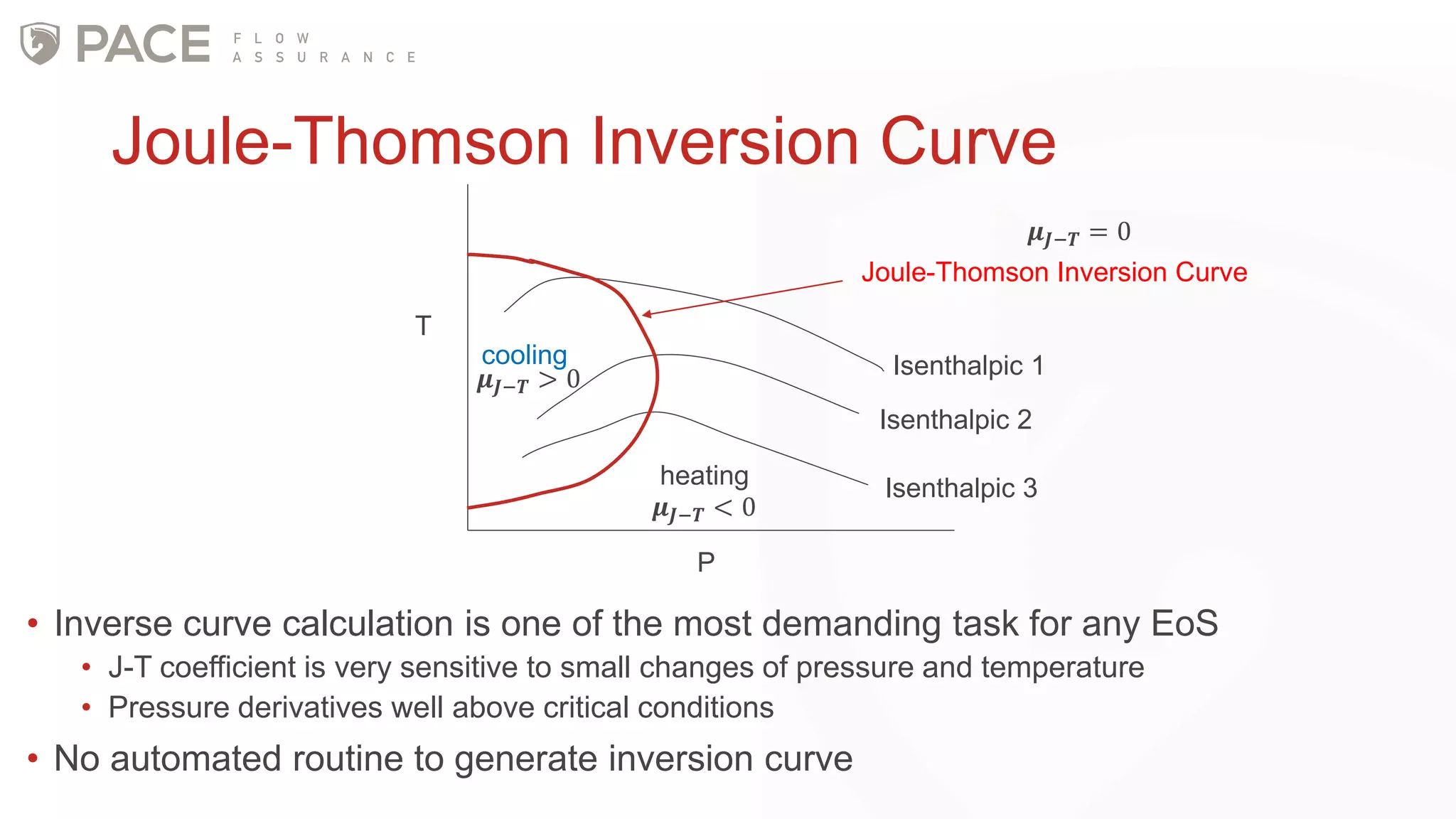
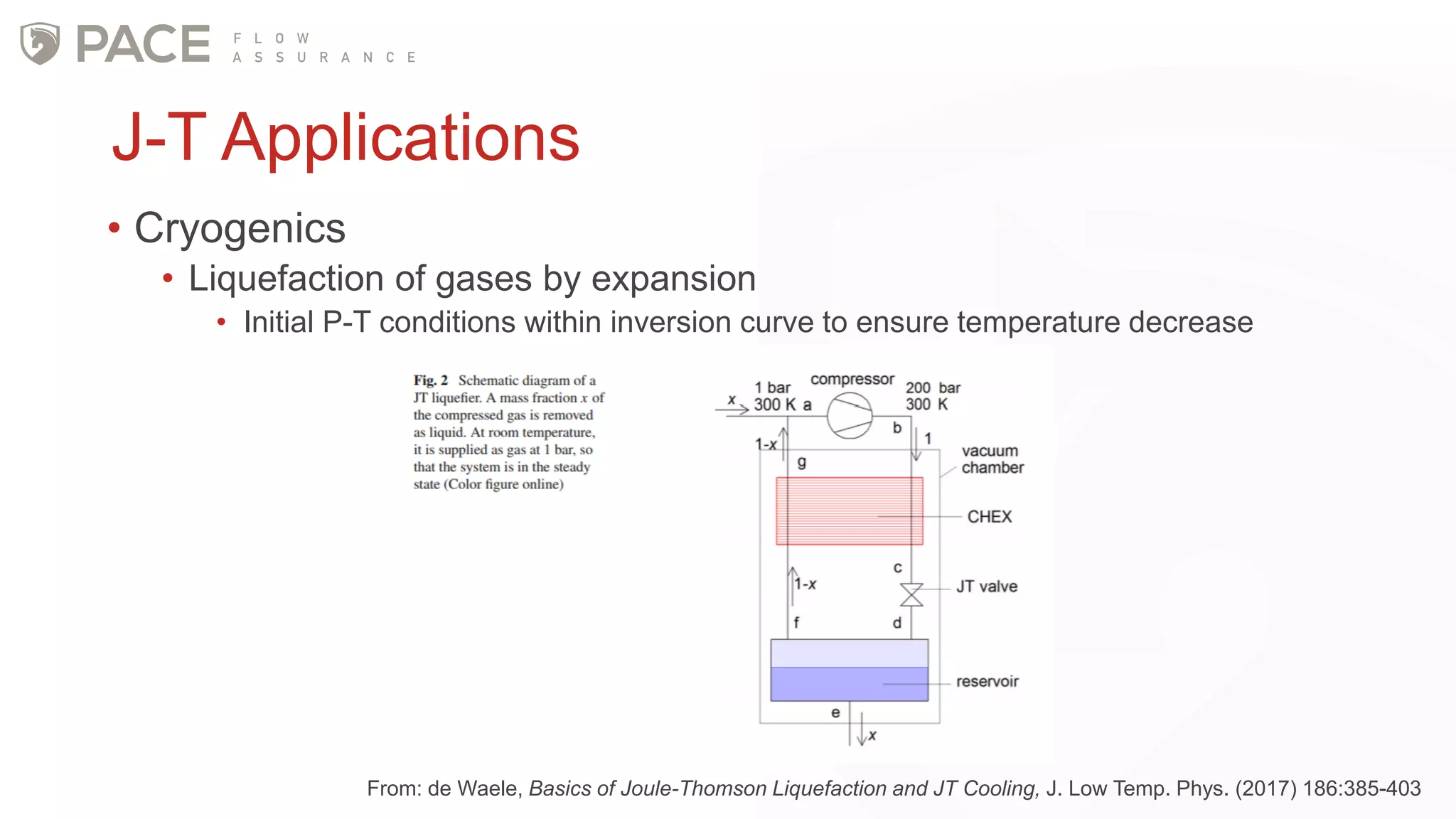
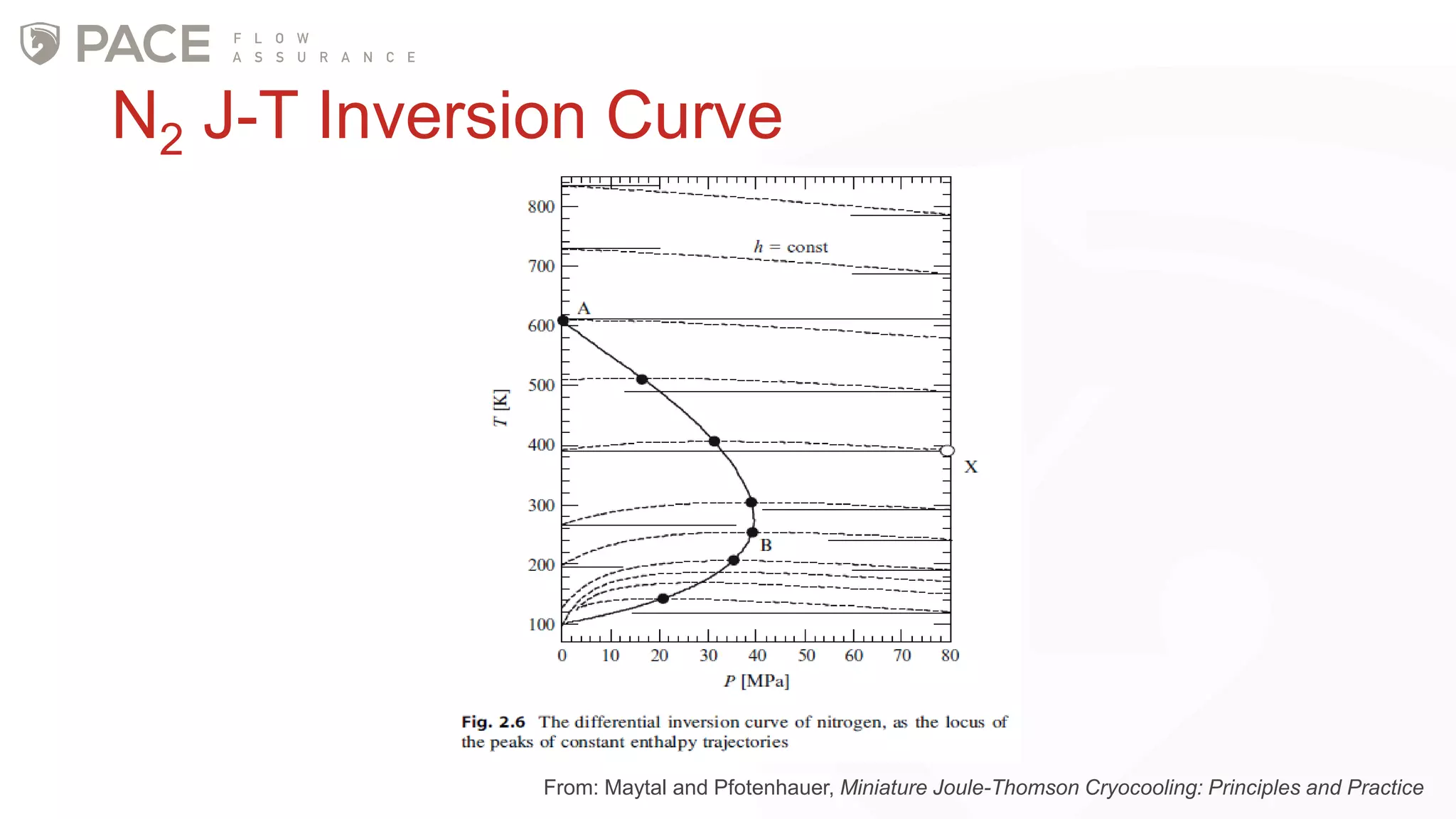
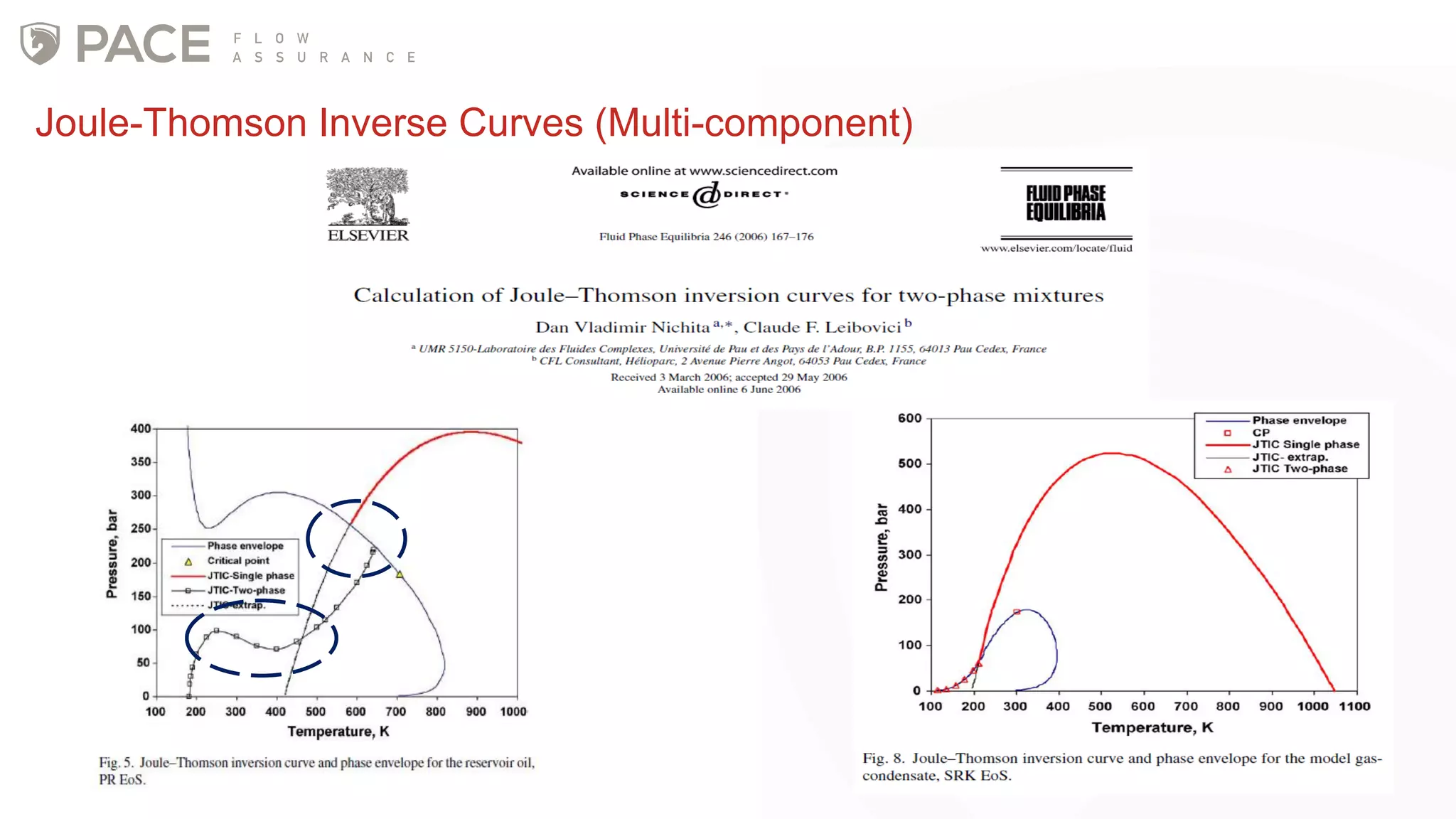
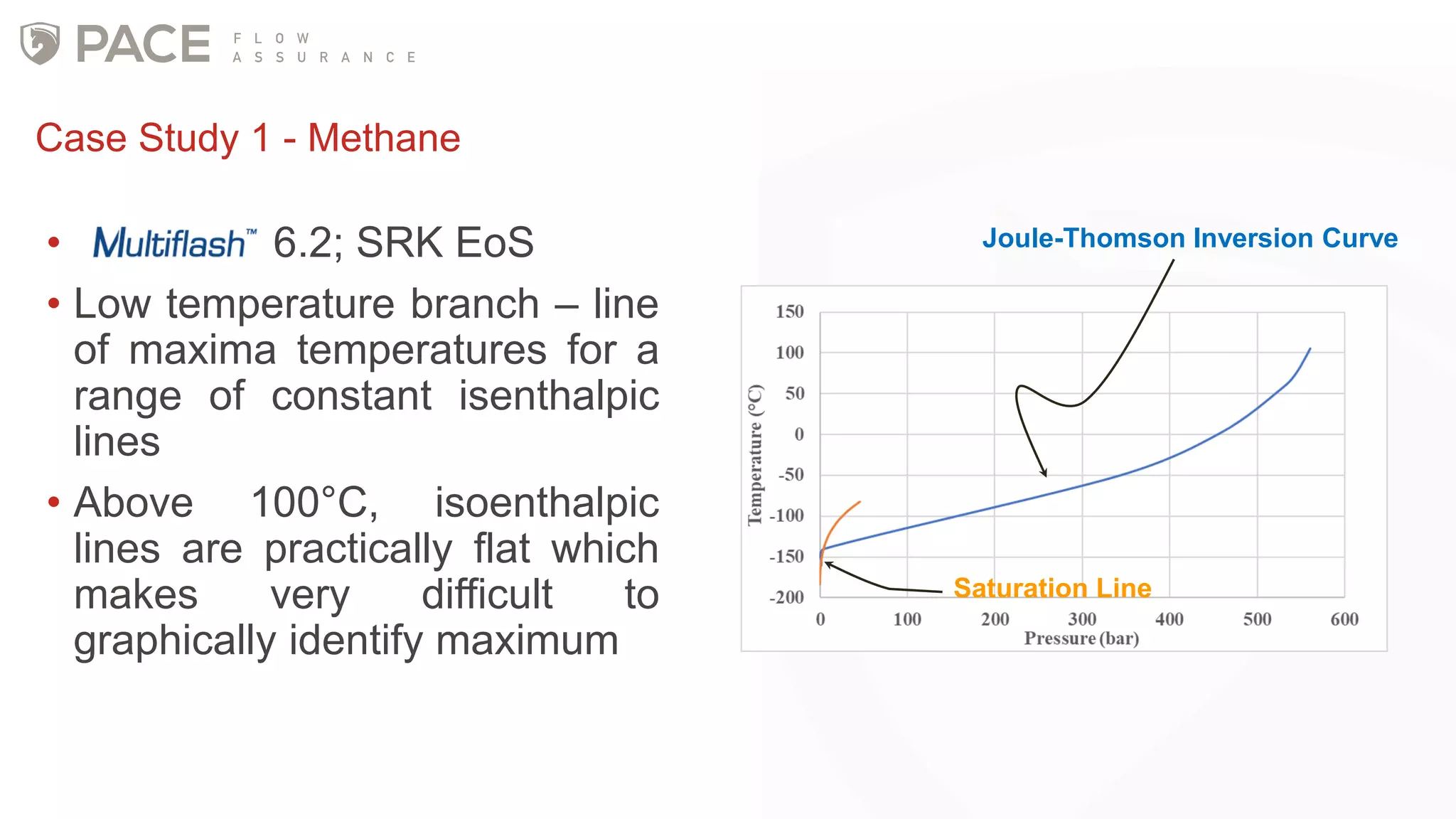
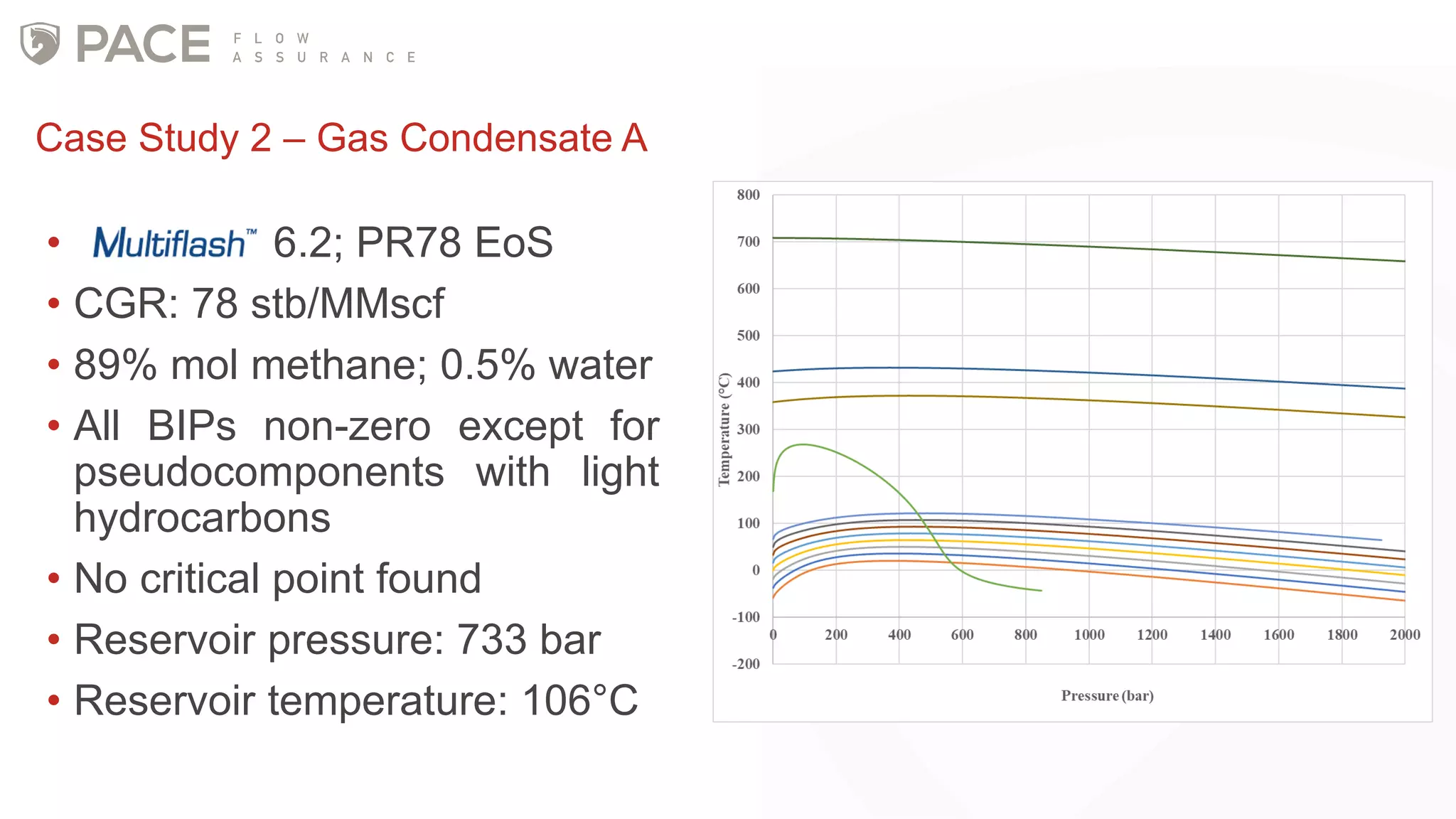
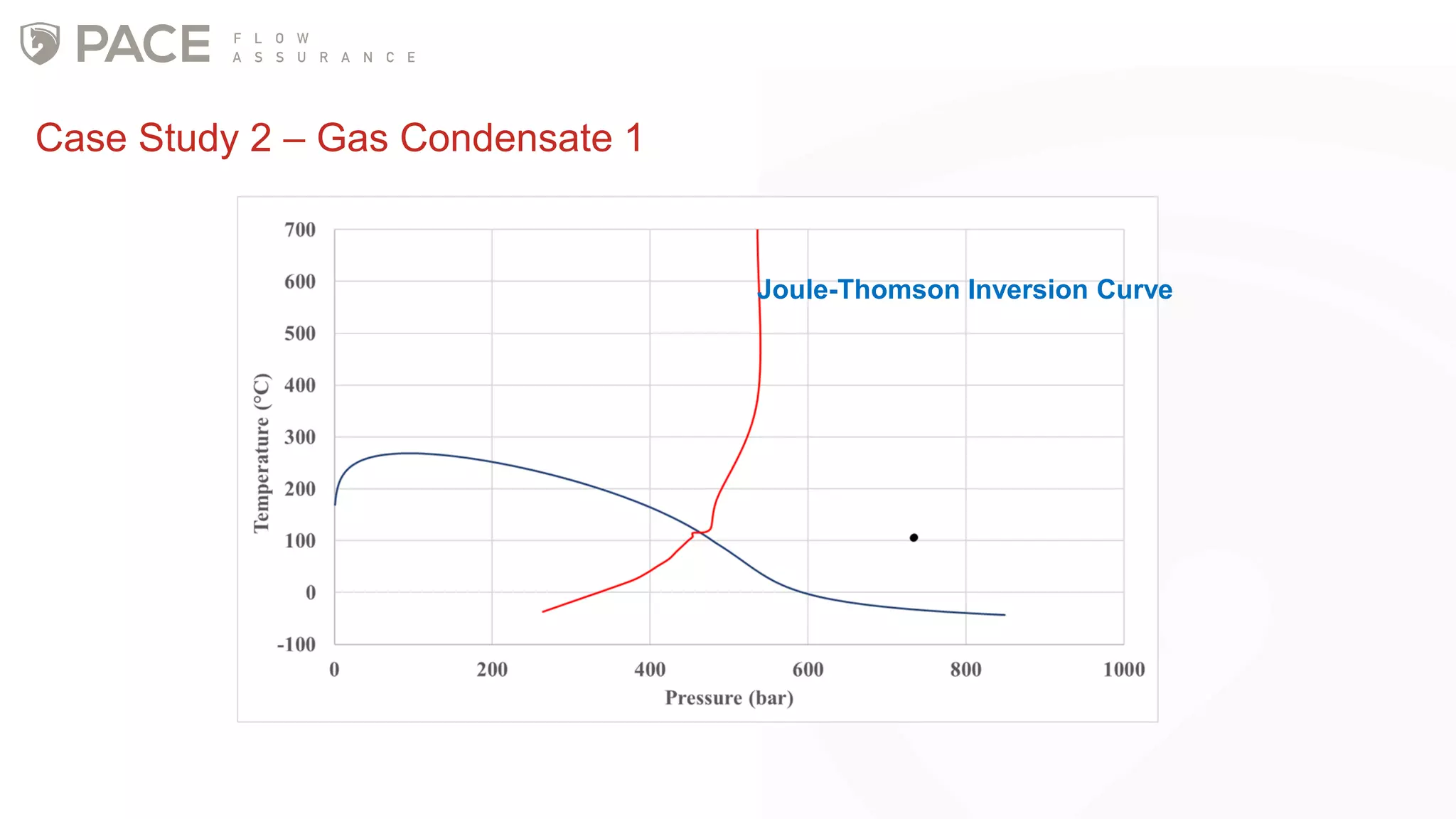
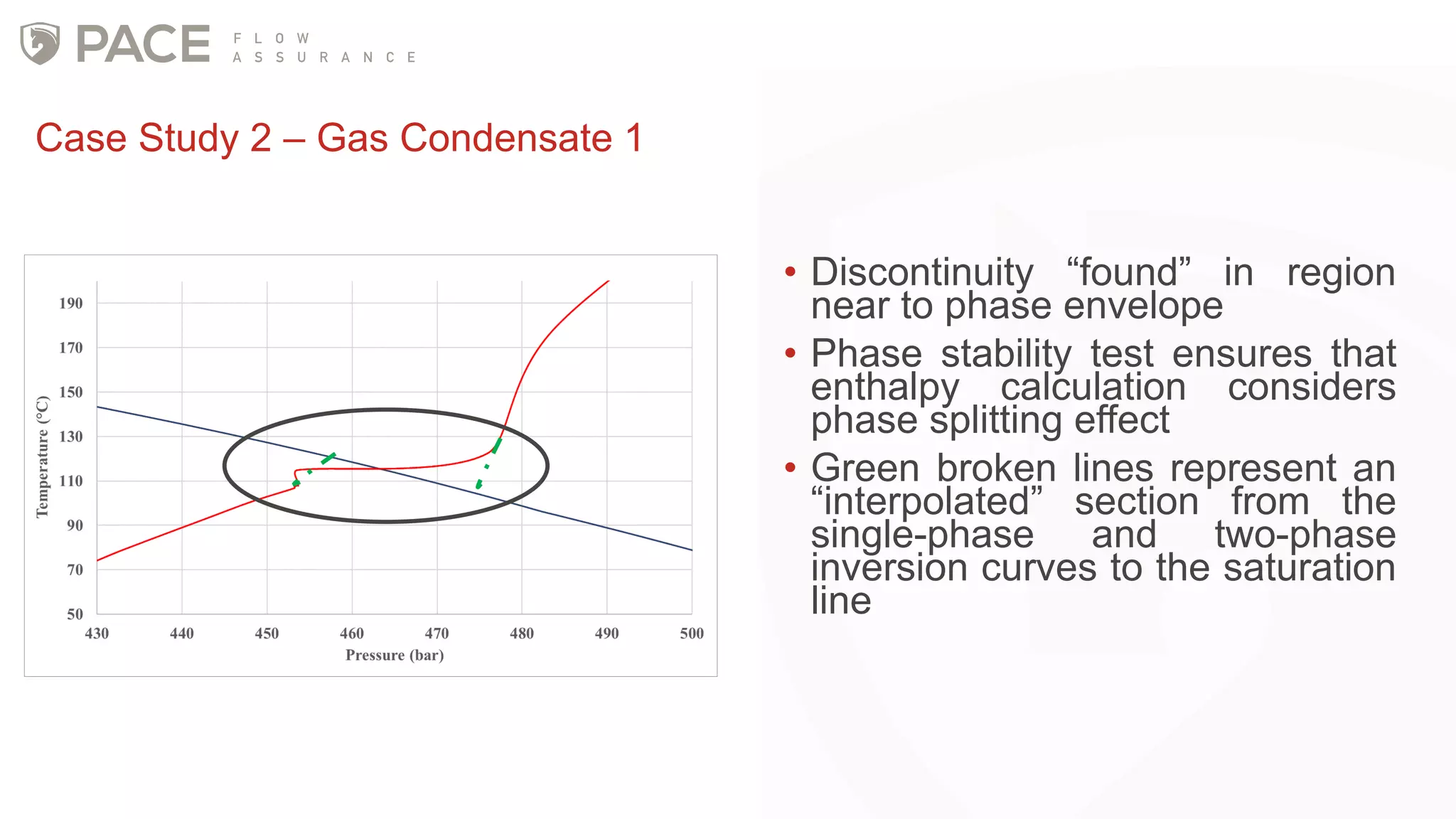
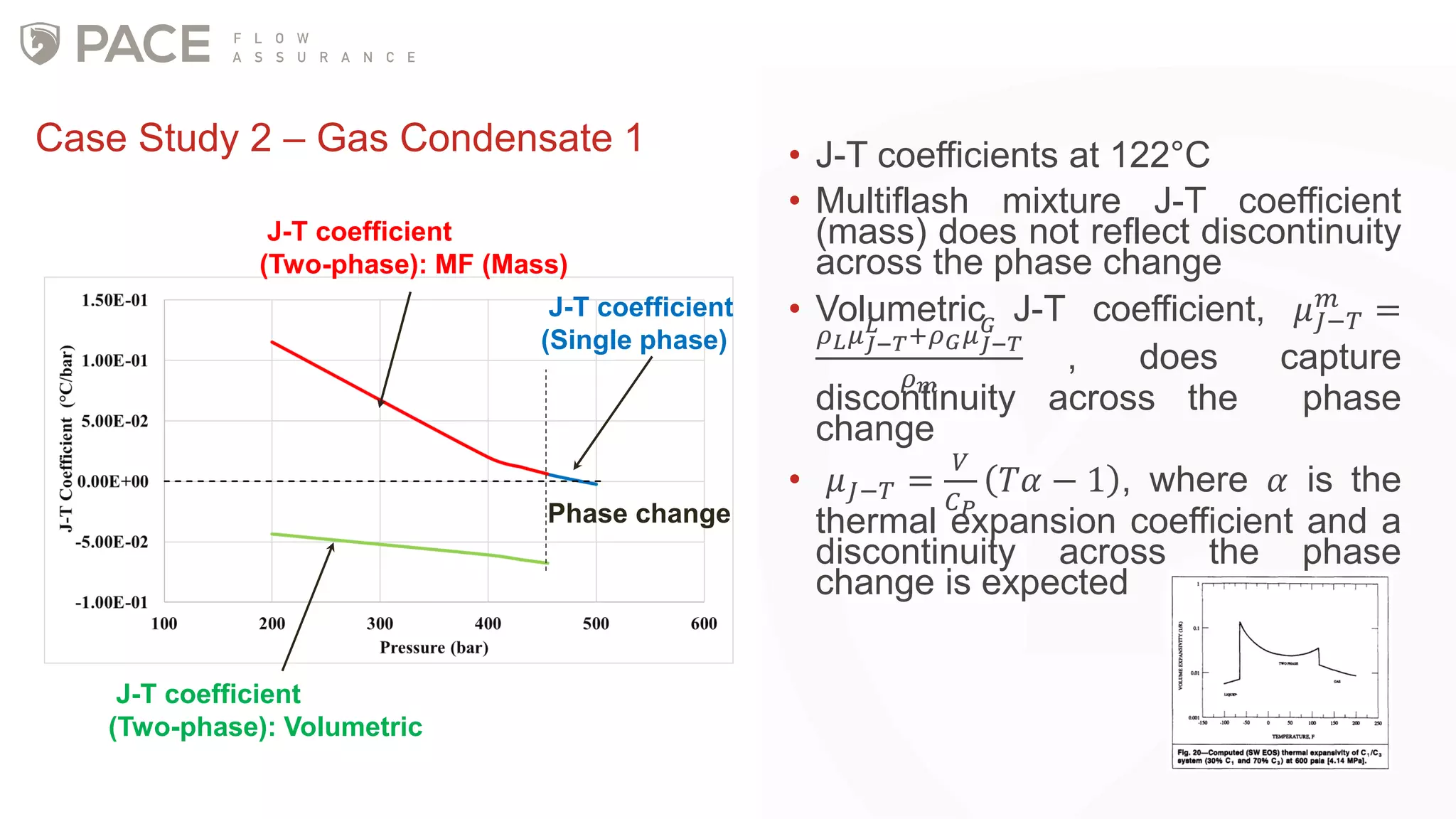
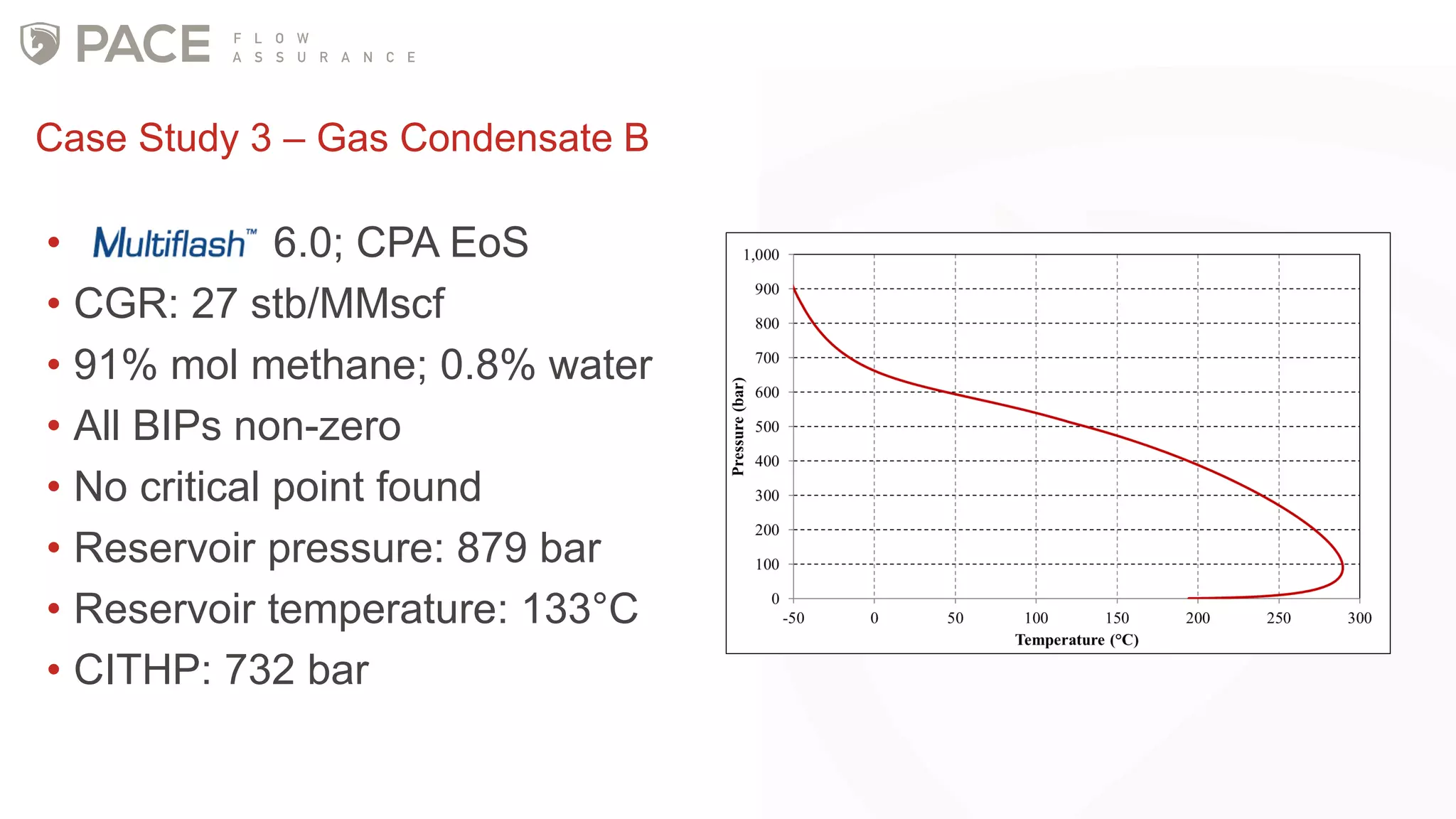
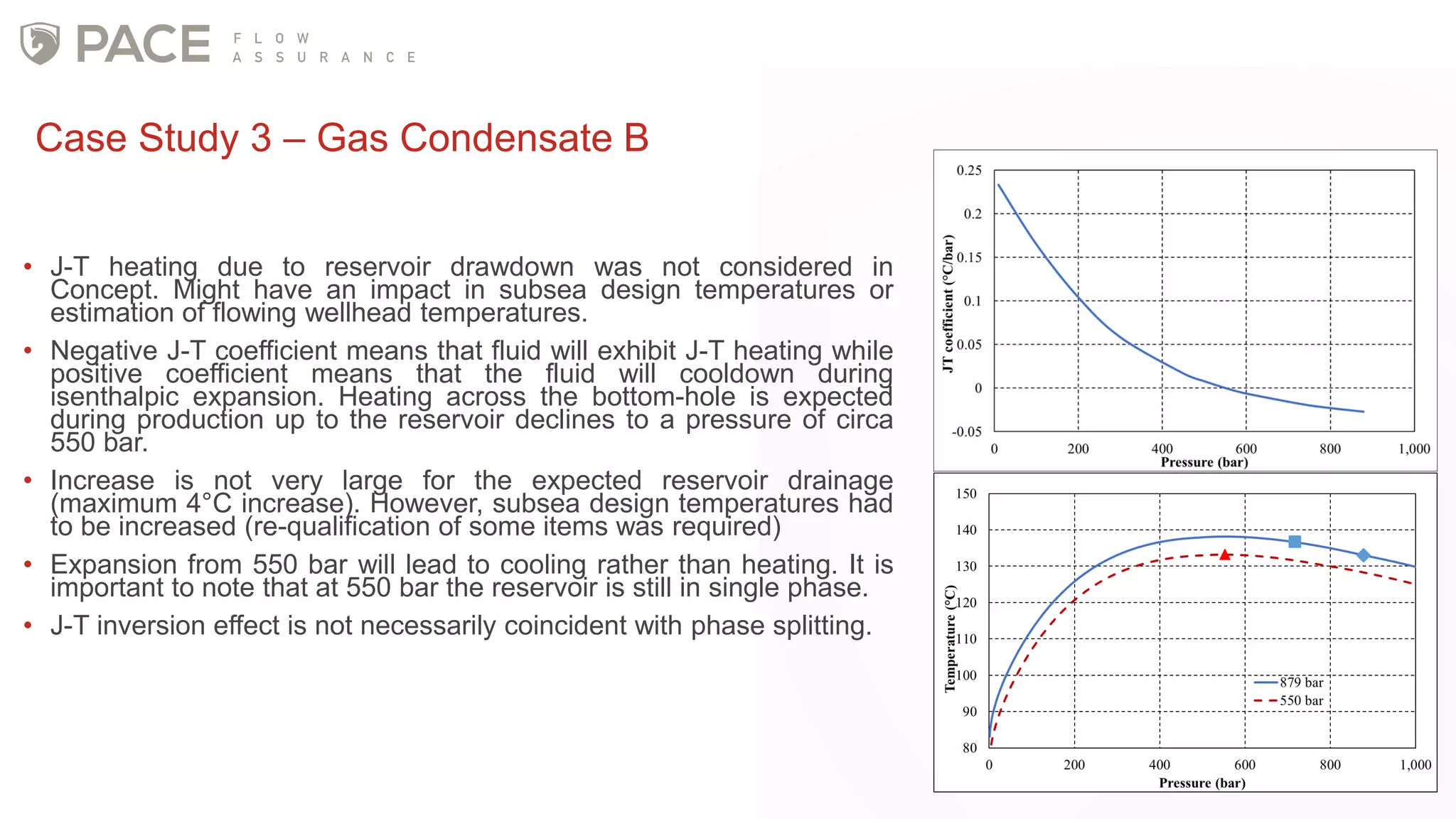
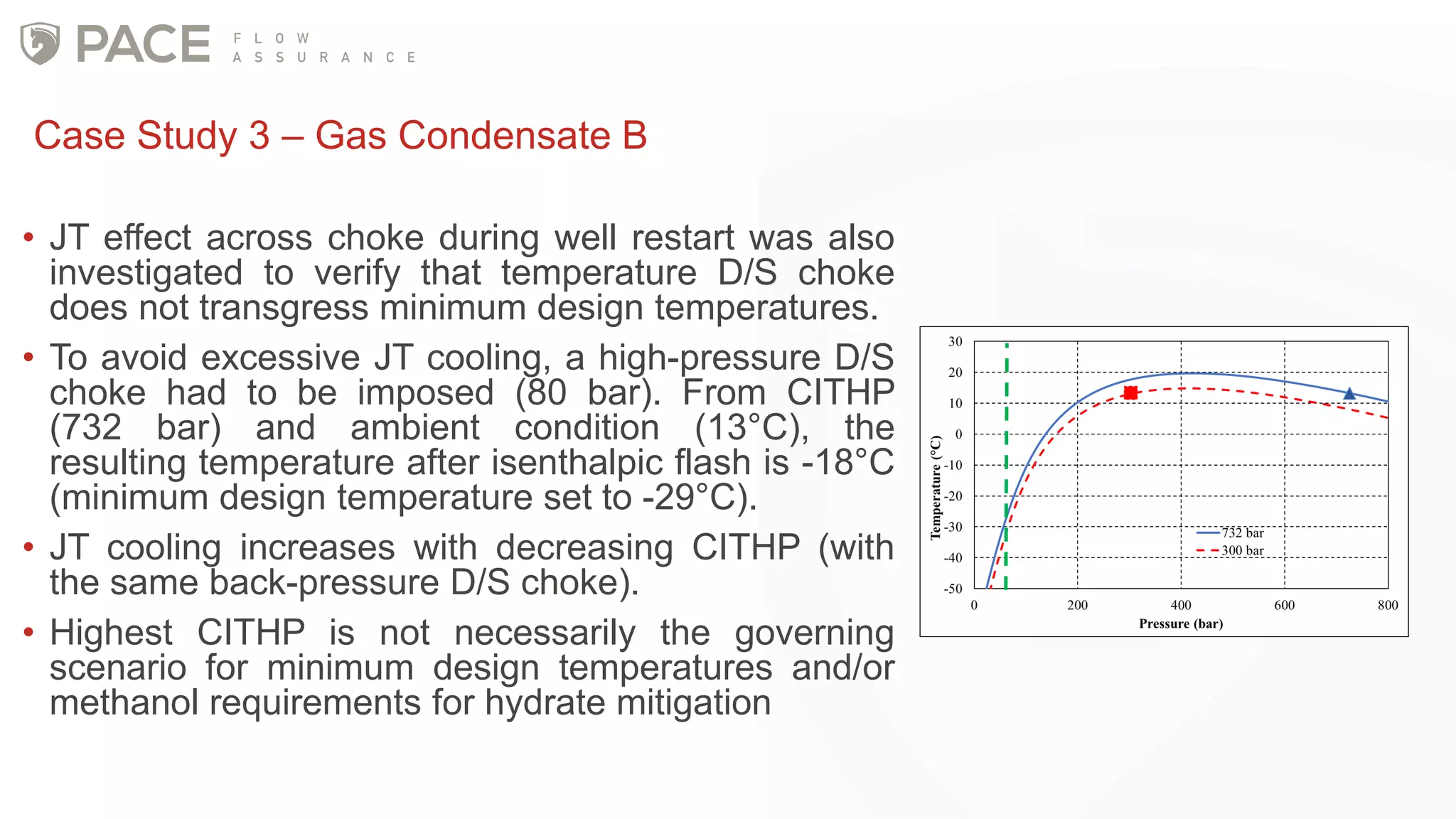
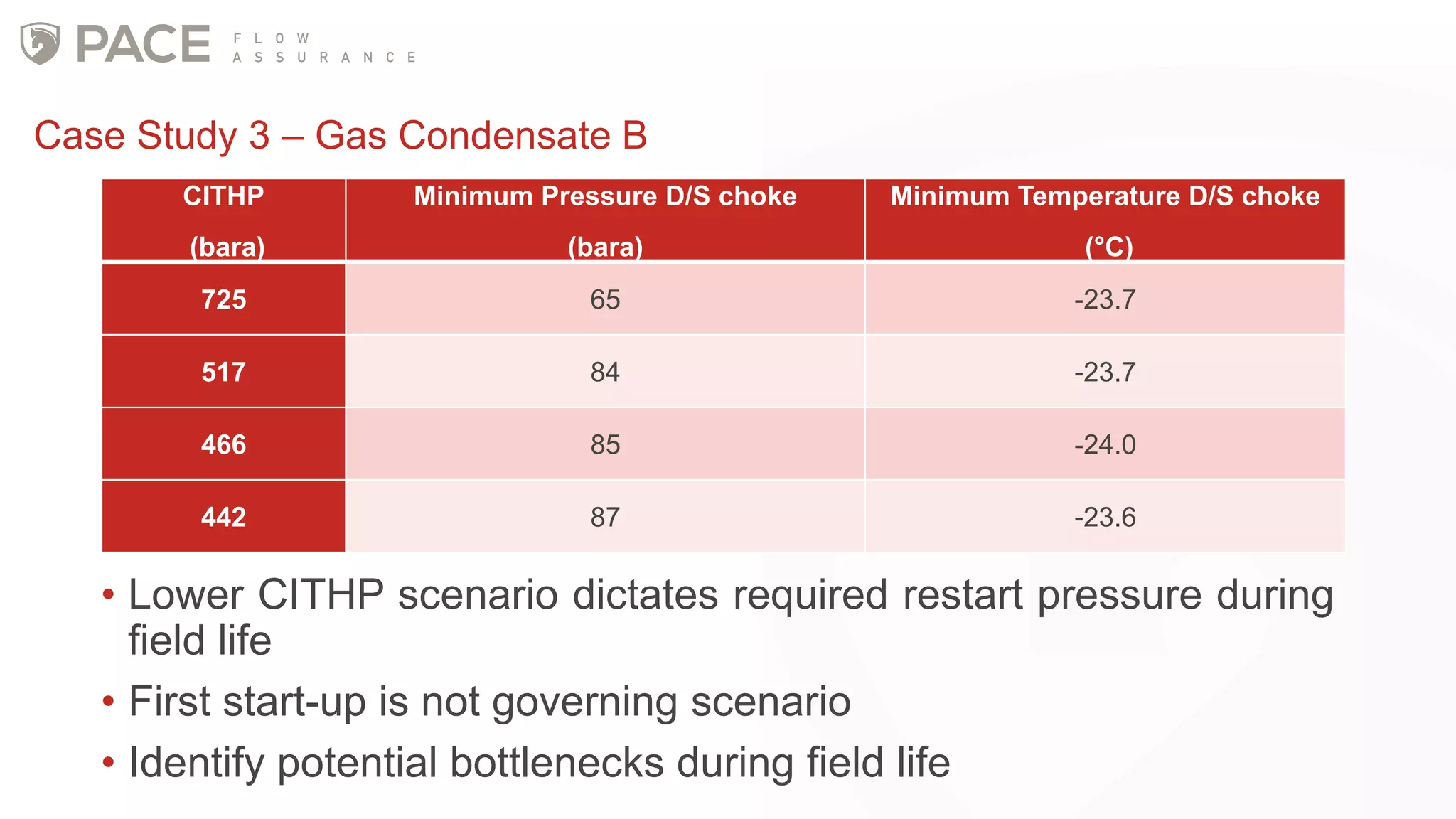
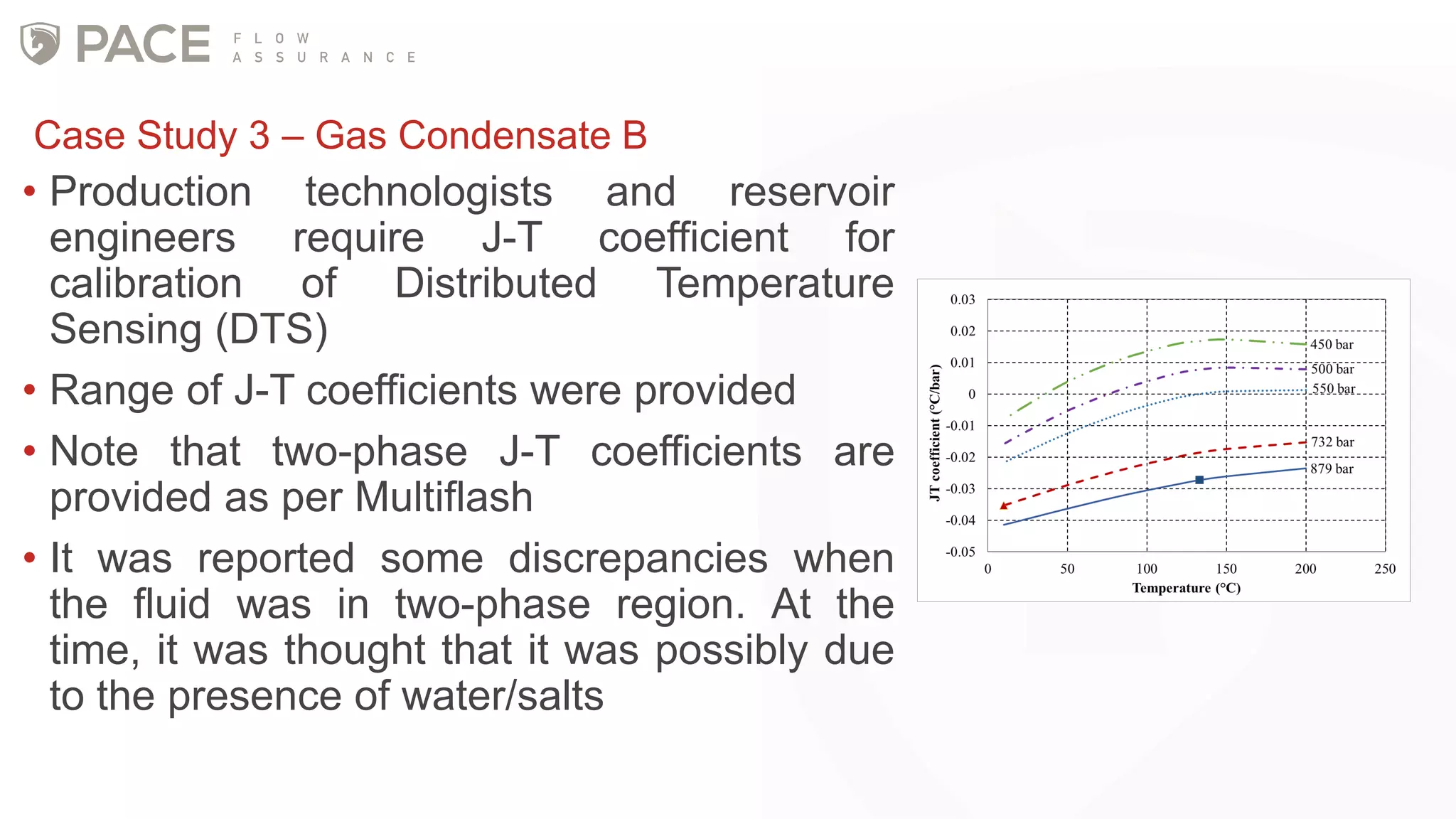
The document discusses the Joule-Thomson effect and its implications for engineering design in high-pressure, high-temperature oil and gas reservoirs. It provides an overview of the Joule-Thomson effect and coefficient, and presents case studies analyzing the Joule-Thomson inversion curves for different gas condensate mixtures using various equations of state. The case studies demonstrate how accounting for the Joule-Thomson effect is important for determining design temperatures and pressures during operations like reservoir drawdown and well start-ups.