This document summarizes key concepts in biochemistry, including:
1) It describes different types of energy like chemical, electrical, and mechanical energy and how they relate to living systems and concepts of potential and kinetic energy.
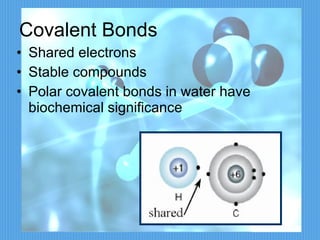
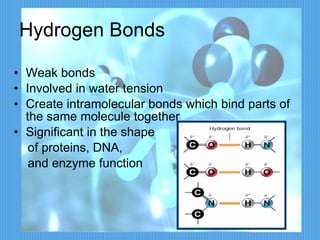
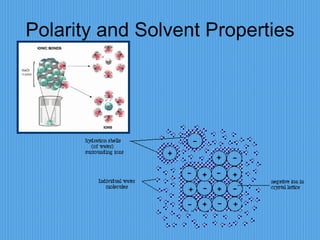
2) It discusses important chemical bonds for life like ionic bonds between atoms, covalent bonds involving shared electrons, and hydrogen bonds that influence molecular structure.
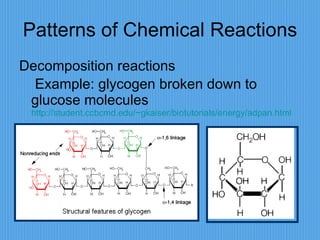
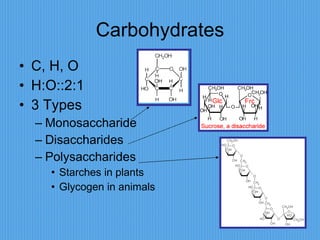
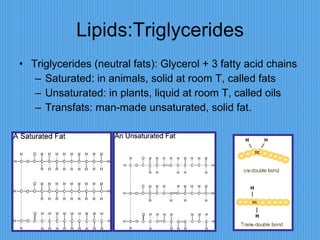
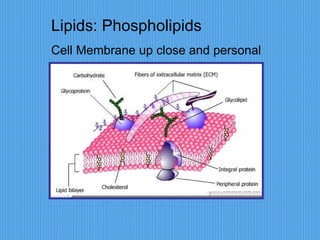
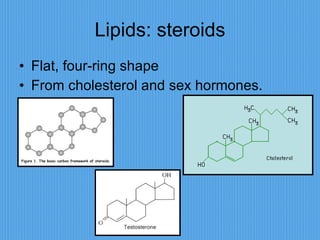
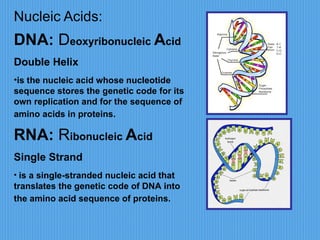
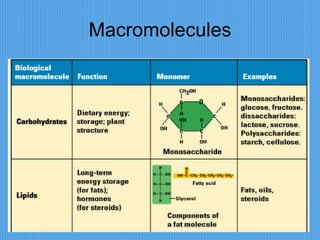
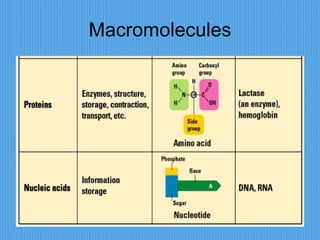
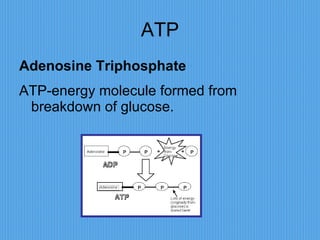
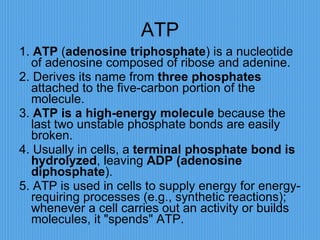
3) It outlines major organic macromolecules that make up living things like carbohydrates, lipids, proteins, nucleic acids, and ATP and their biological roles and significance.