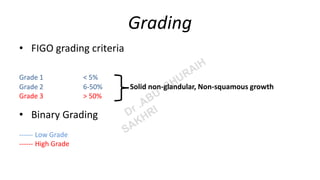
Endometrioid carcinoma of the uterine corpus is a malignant epithelial neoplasm characterized by varying architectural patterns and is predominantly found in postmenopausal women, with a high incidence in high human development index countries. The carcinoma is classified into four molecular subtypes, with clinical features often presenting as postmenopausal bleeding, and an alignment of pathogenesis with prolonged estrogen exposure and genetic syndromes like Lynch syndrome. Histopathological assessment, including grading per FIGO criteria, and immunohistochemistry are crucial for differentiating subtypes and informing prognosis and treatment strategies.