Electron microscopy and production of antisera can be used to detect and identify plant viruses.
1) Electron microscopy can detect virus particles in infected plant tissue and identify unknown viruses. It is often combined with antisera in a technique called immuno electron microscopy.
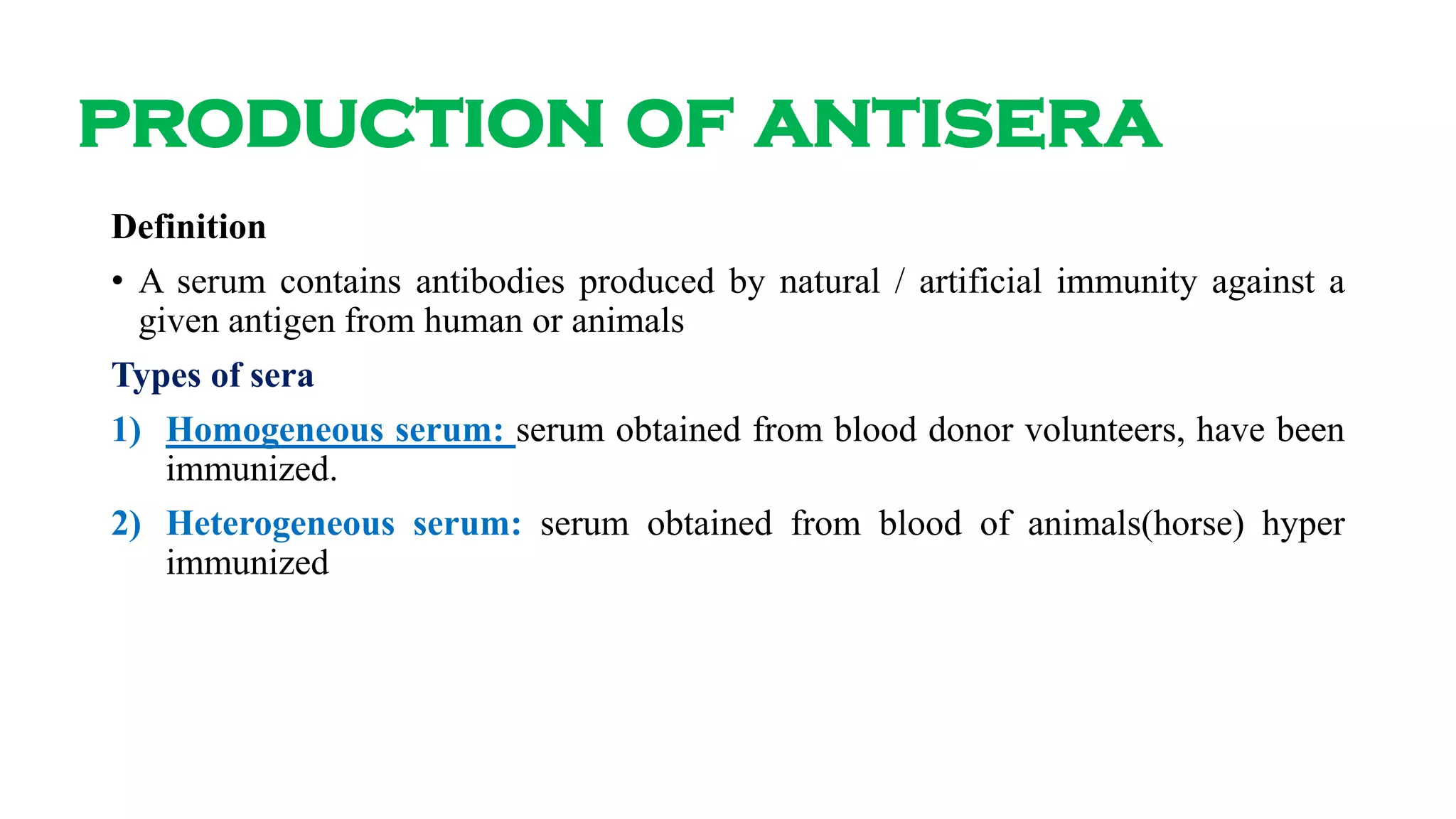
2) Antisera are produced by injecting purified antigens into rabbits to stimulate an immune response and antibody production. Test bleedings are taken from the rabbits and the resulting antisera are evaluated for avidity, specificity, and titer against the target antigen.