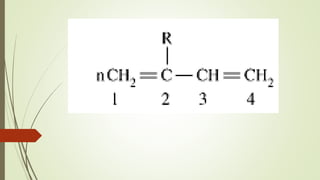
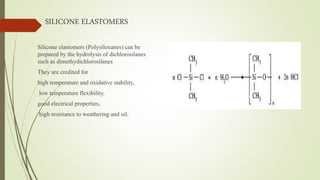
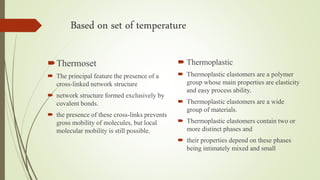
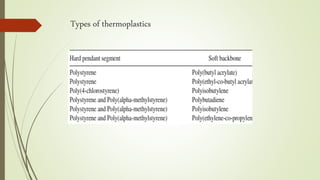
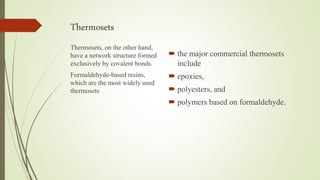
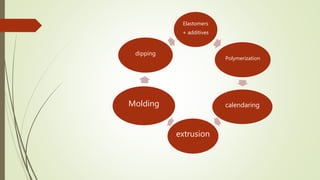
The document discusses elastomeric polymers, detailing their properties, types, manufacturing processes, and applications. Elastomers, known for their elastic recovery and versatility, include natural and synthetic rubbers with numerous applications ranging from tires to footwear. Future trends highlight innovative uses of elastomers in advanced technologies like magnetorheological and electrorheological elastomers.