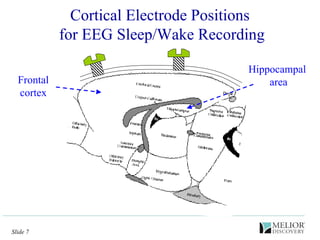
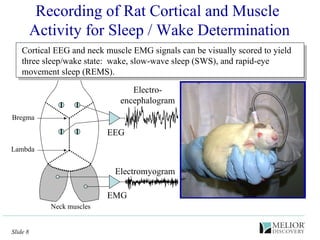
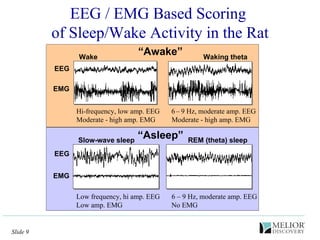
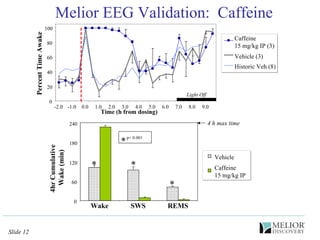
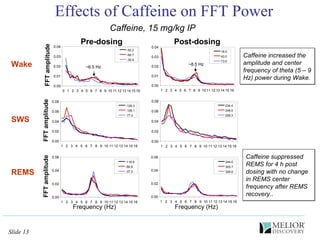
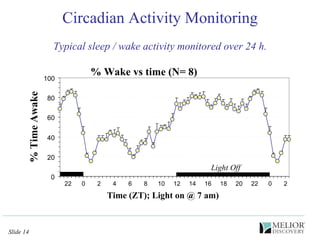
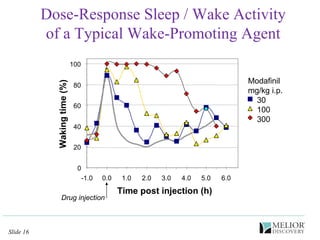
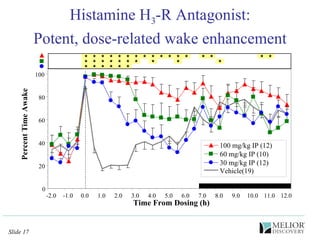
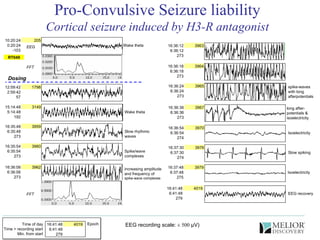
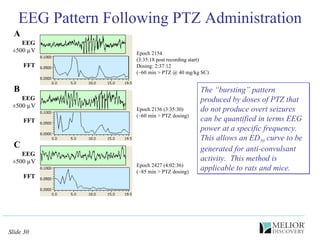
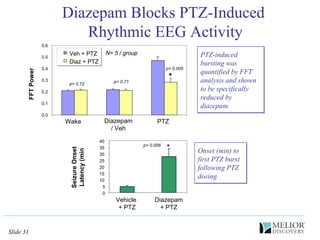
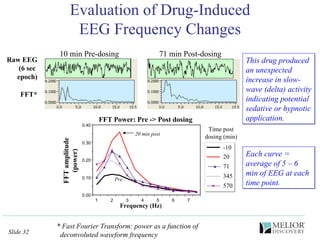
The document discusses the use of electrophysiological models to analyze sleep/wake activity and EEG in rodents, particularly rats and mice, as a means to evaluate neurological disorders and drug efficacy. It highlights various evaluation methods, including drug interactions, physiological impacts, and the role of EEG measures in characterizing sleep states and seizure activity. The sensitivity of EEG recordings allows for detailed quantitative analysis of CNS activity and the effectiveness of wake-promoting agents.








![THE END Publications Le S, JA Gruner, JR M athiasen, MJ Marino, and H Schaffhauser. (2008). Correlation between ex vivo receptor occupancy and wake promoting activity of selective H3 receptor antagonists. J Pharmacol Exp Ther. 325:902-9. Fiocchi EM, YG Lin, L Aimone, JA Gruner, DG Flood. (2009). Armodafinil promotes wakefulness and activates Fos in rat brain. Pharmacol Biochem Behav. May;92:549-57. Gruner JA, VR Marcy, Y-G Lin, D Bozyczko-Coyne, MJ Marino, M Gasior. 2009. The Roles of Dopamine Transport Inhibition and Dopamine Release Facilitation in Wake Enhancement and Rebound Hypersomnolence Induced by Dopaminergic Agents. Sleep 32:1425-1438. Gruner JA, JR Mathiasen, DG Flood, MJ Marino, M Gasior. 2011. Biochemical, pharmacological, and behavioral characterization of the dopaminergic stimulant sydnocarb in rats. J. Pharmacol. Expt. Ther.. 337:380-90. Hudkins RL, Raddatz R, Tao M, Mathiasen JR, Aimone LD, Becknell NC, Prouty CP, Knutsen LJ, Yazdanian M, Moachon G, Ator MA, Mallamo JP, Marino MJ, Bacon ER, Williams M. 2011. Discovery and Characterization of 6-{4-[3-(R)-2-Methylpyrrolidin-1-yl)propoxy]phenyl}-2H-pyridazin-3-one (CEP-26401, Irdabisant): A Potent, Selective Histamine H(3) Receptor Inverse Agonist. J Med Chem. 54:4781-92. Papers in progress Gruner JA, VR Marcy, Y-G Lin, MJ Marino. The Relative Efficacies of Caffeine, Amphetamine, and Modafinil in the Presence and Absence of Sleep Deprivation in the Rat. 2009. ( In preparation ) Raddatz R, RL Hudkins, JR Mathiasen, JA Gruner, S Le, H Schaffhauser, D Bozyczko-Coyne, MJ Marino, MA Ator, ER Bacon, JP Mallamo, M Williams. A potent and selective histamine H3 receptor antagonist/ inverse agonist with cognition-enhancing and wake promoting activities. ( In preparation. ) Gasior M, JA Gruner, VR Marcy, Y-G Lin, MJ Marino. Wake Promoting Effects of Nicotine are Mediated by alpha-4 beta-2 and alpha-7 Subunit-Containing Nicotinic Acetylcholine Receptors in Rats. ( In preparation. )](https://image.slidesharecdn.com/eegsleepwakeevaluation-13113505342604-phpapp02-110722110403-phpapp02/85/Eeg-Sleep-Wake-Evaluation-33-320.jpg)