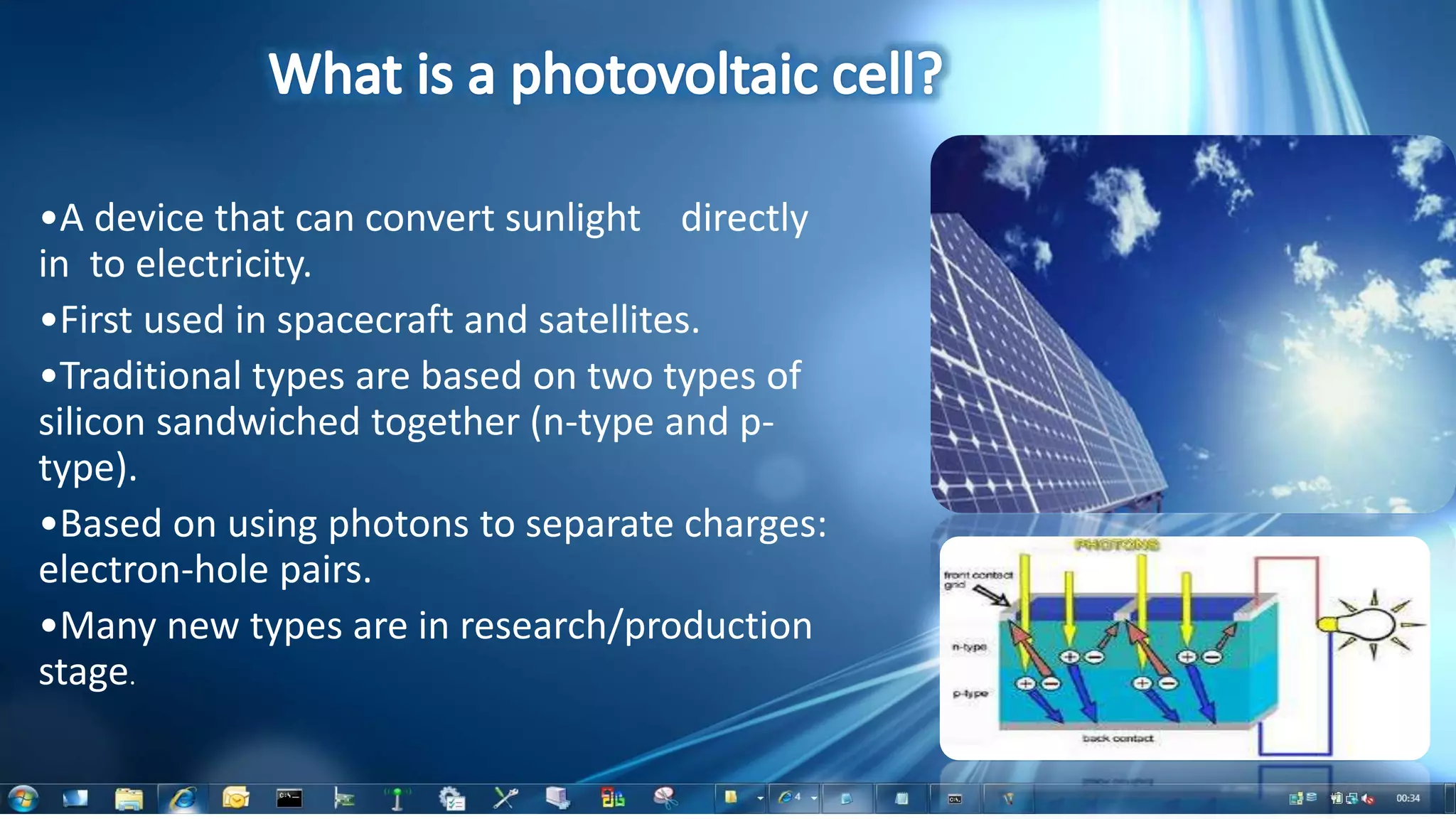
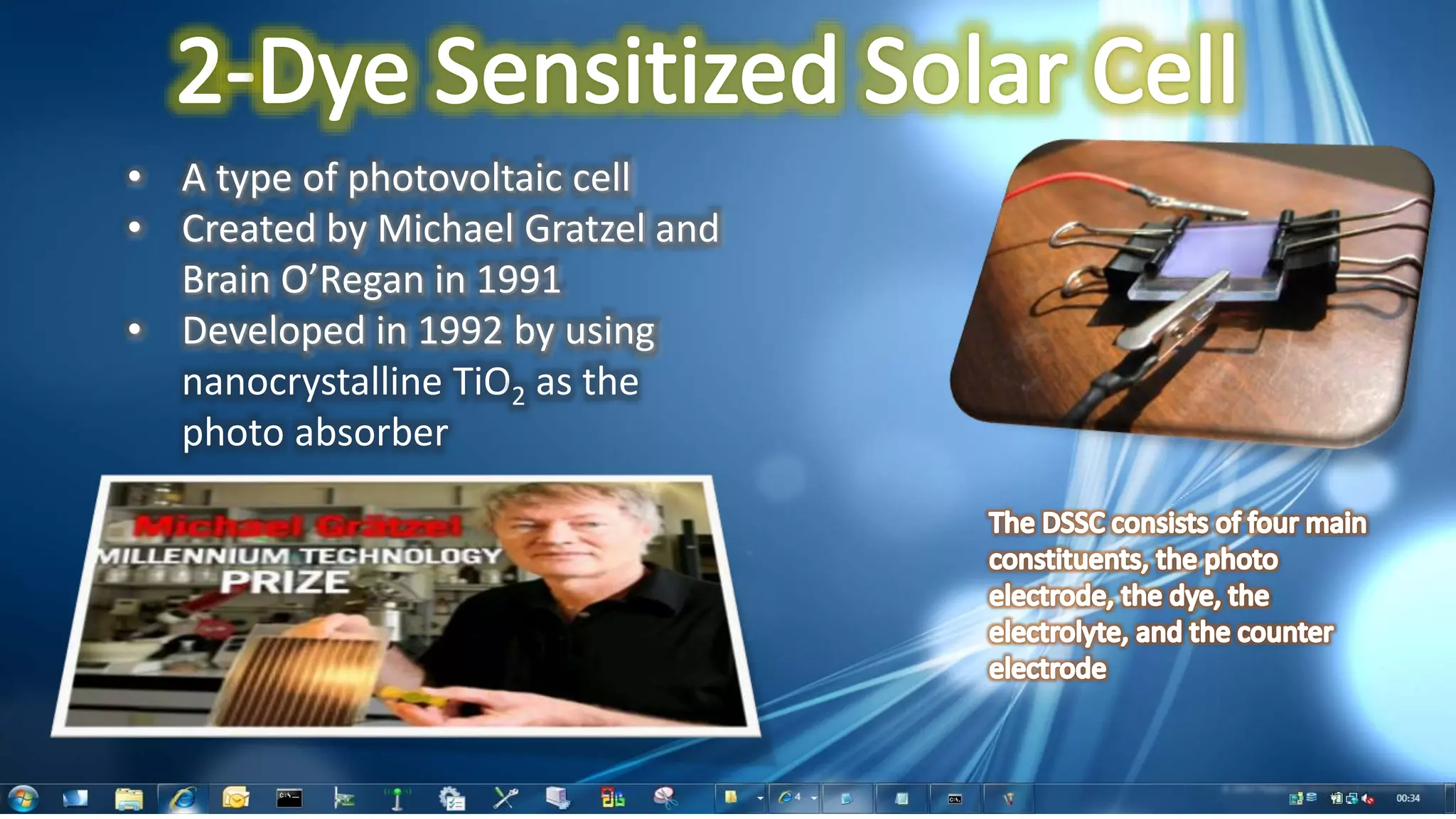
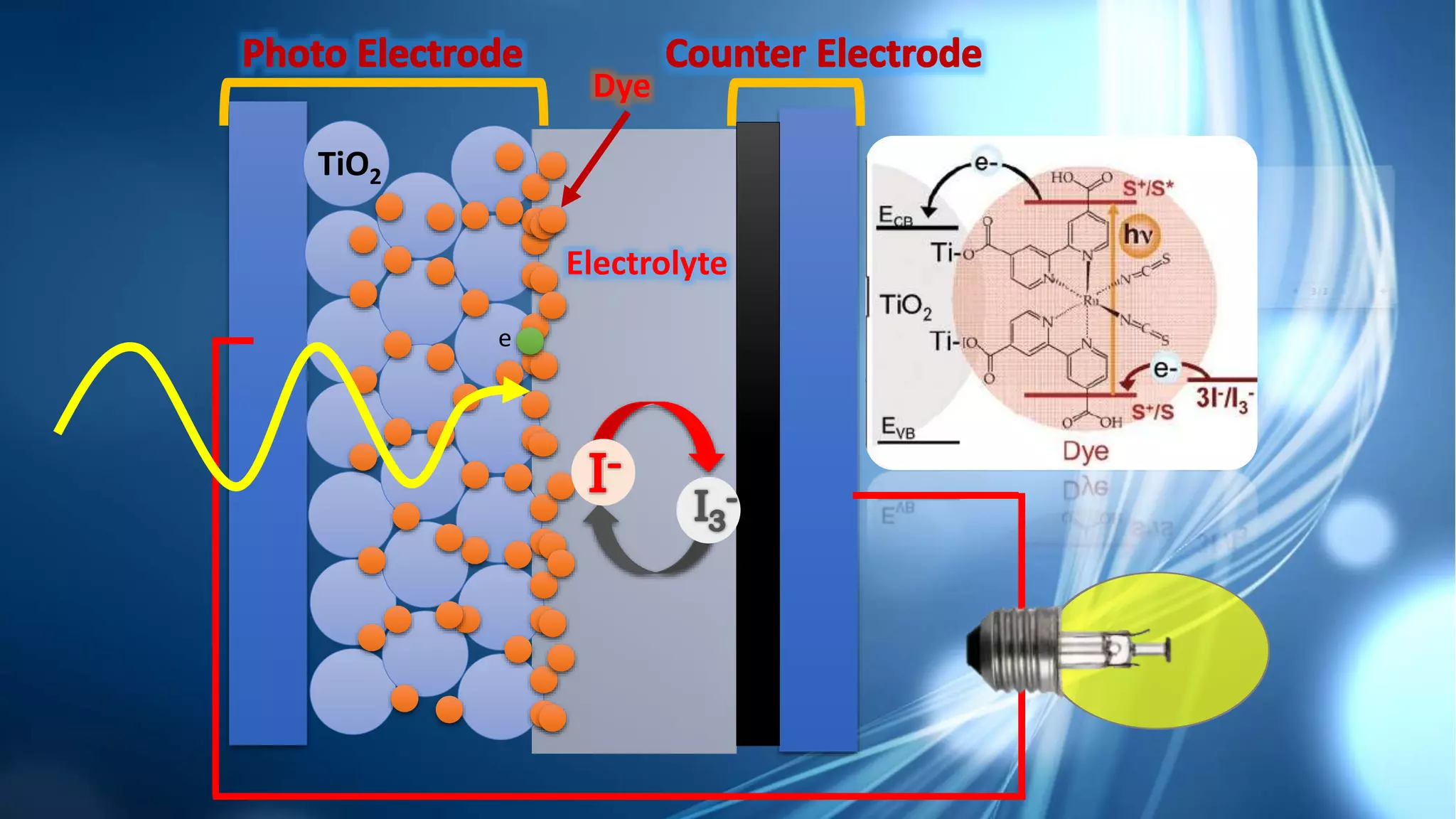
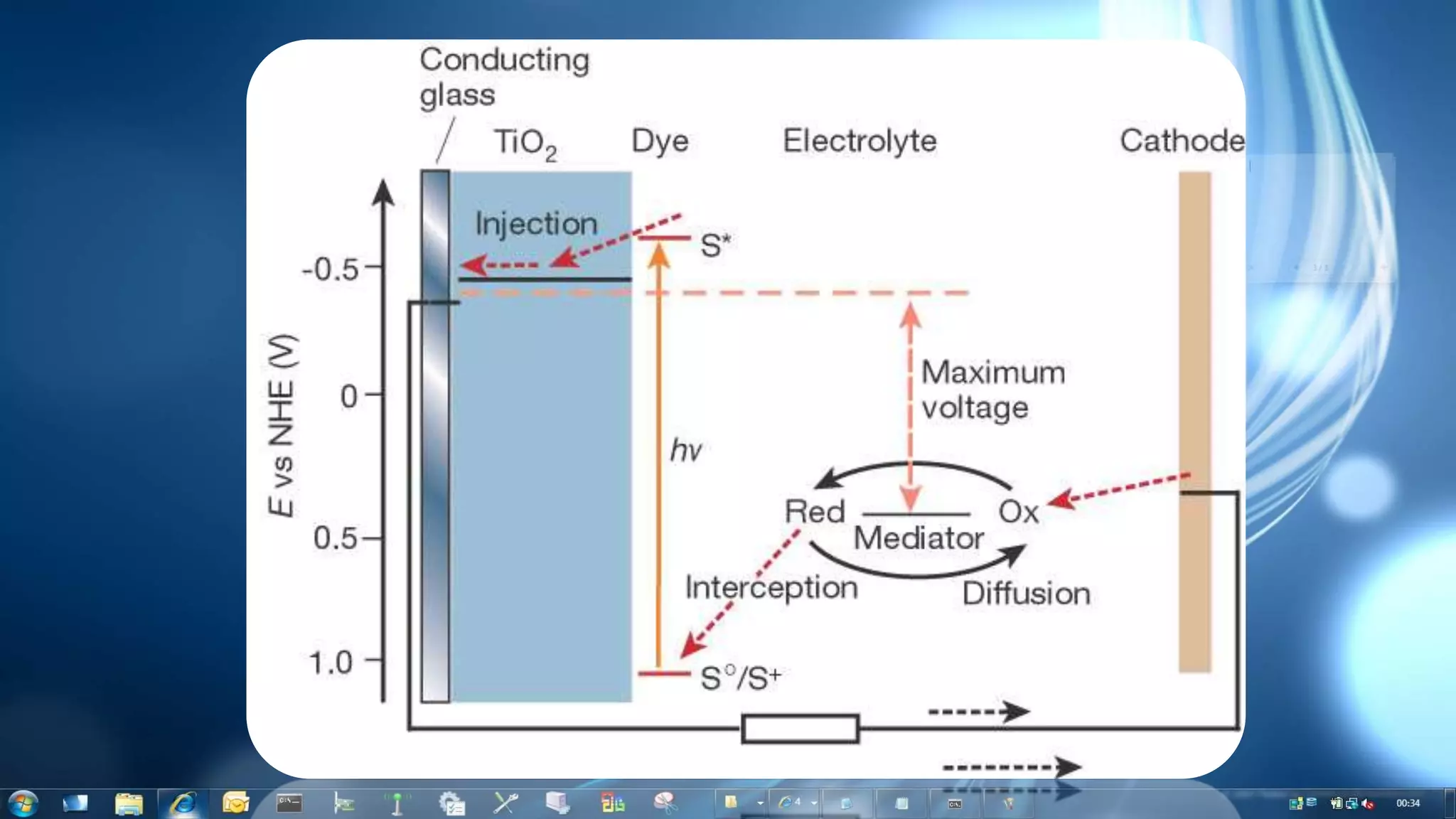
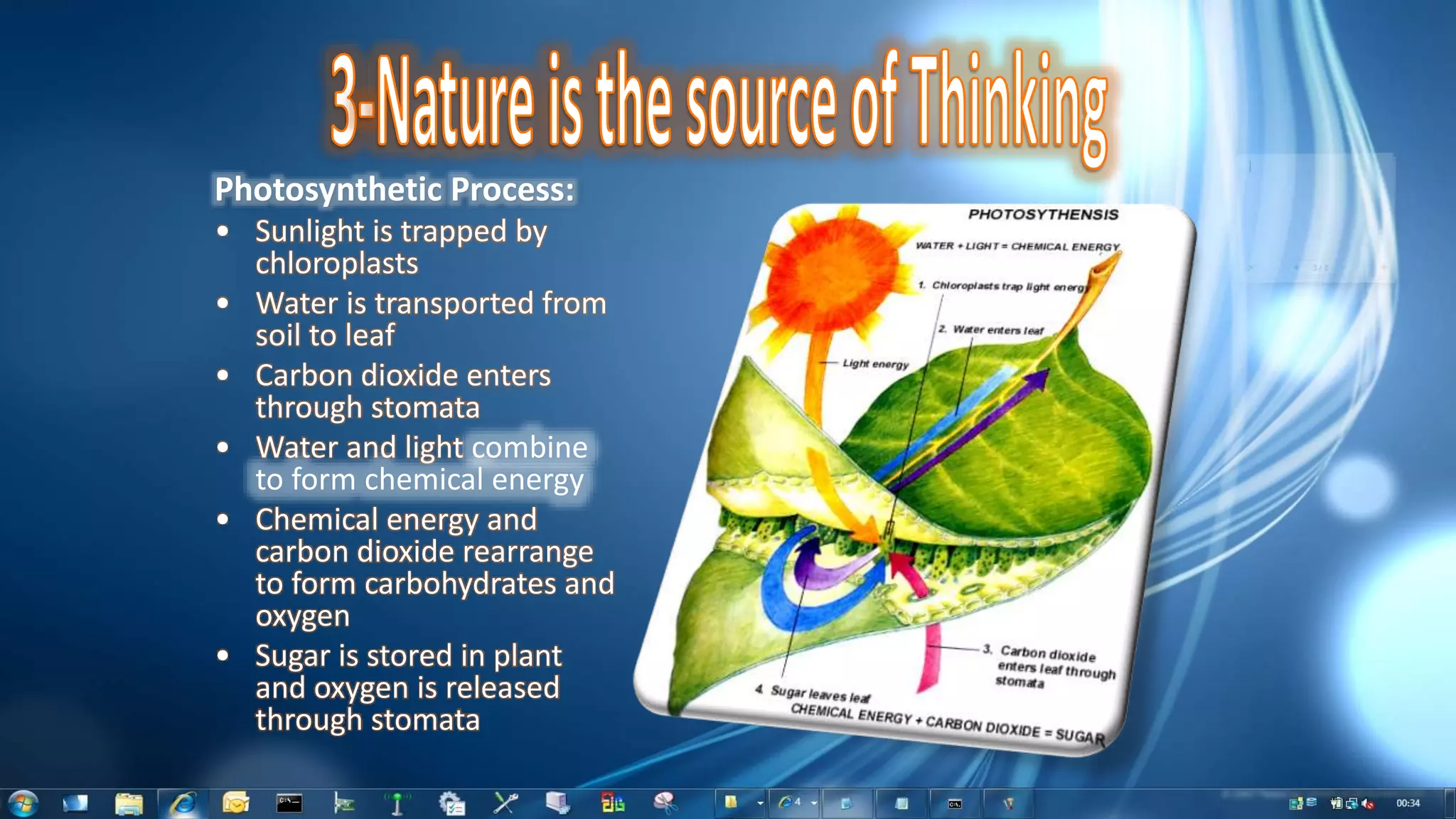
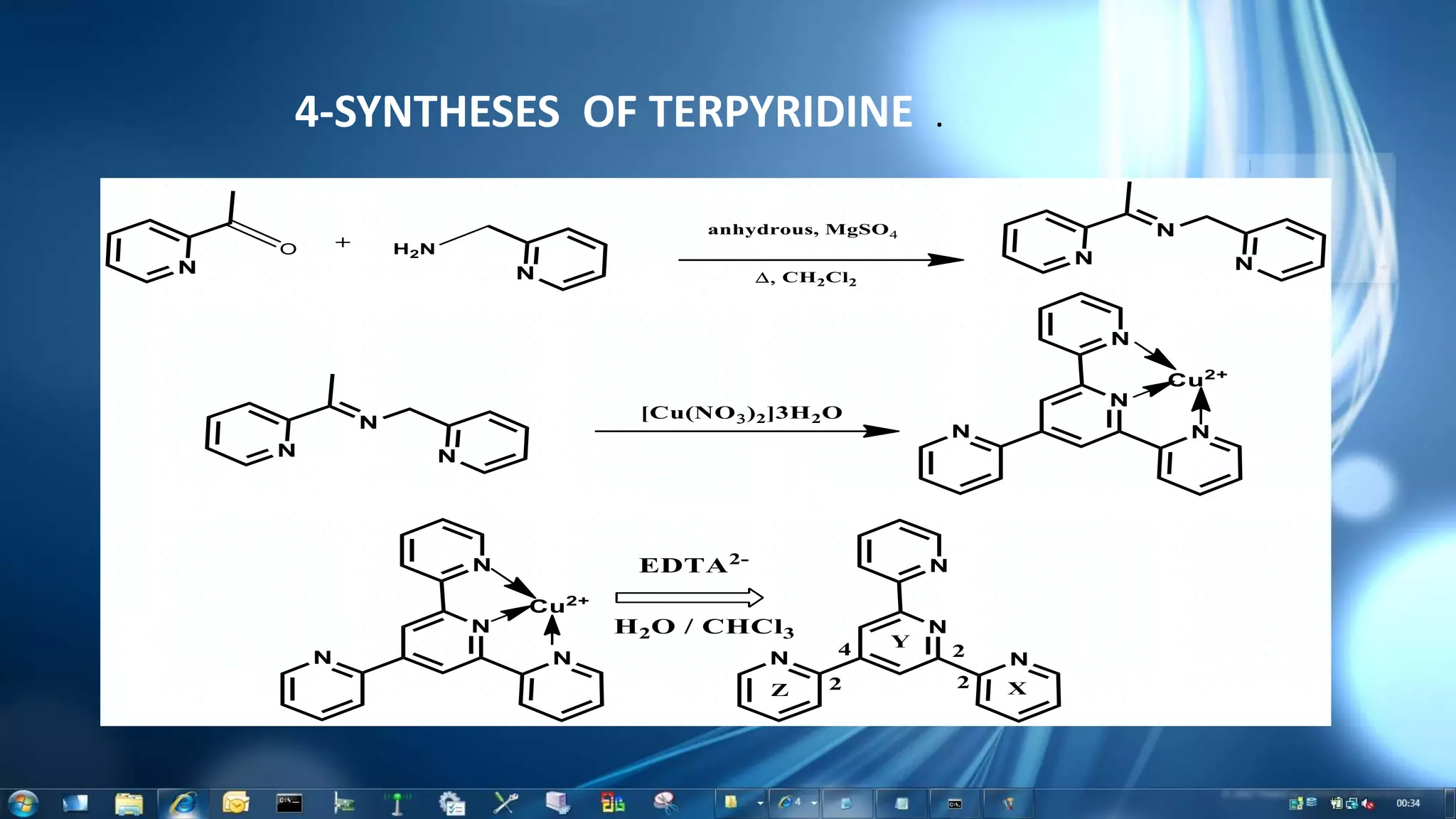
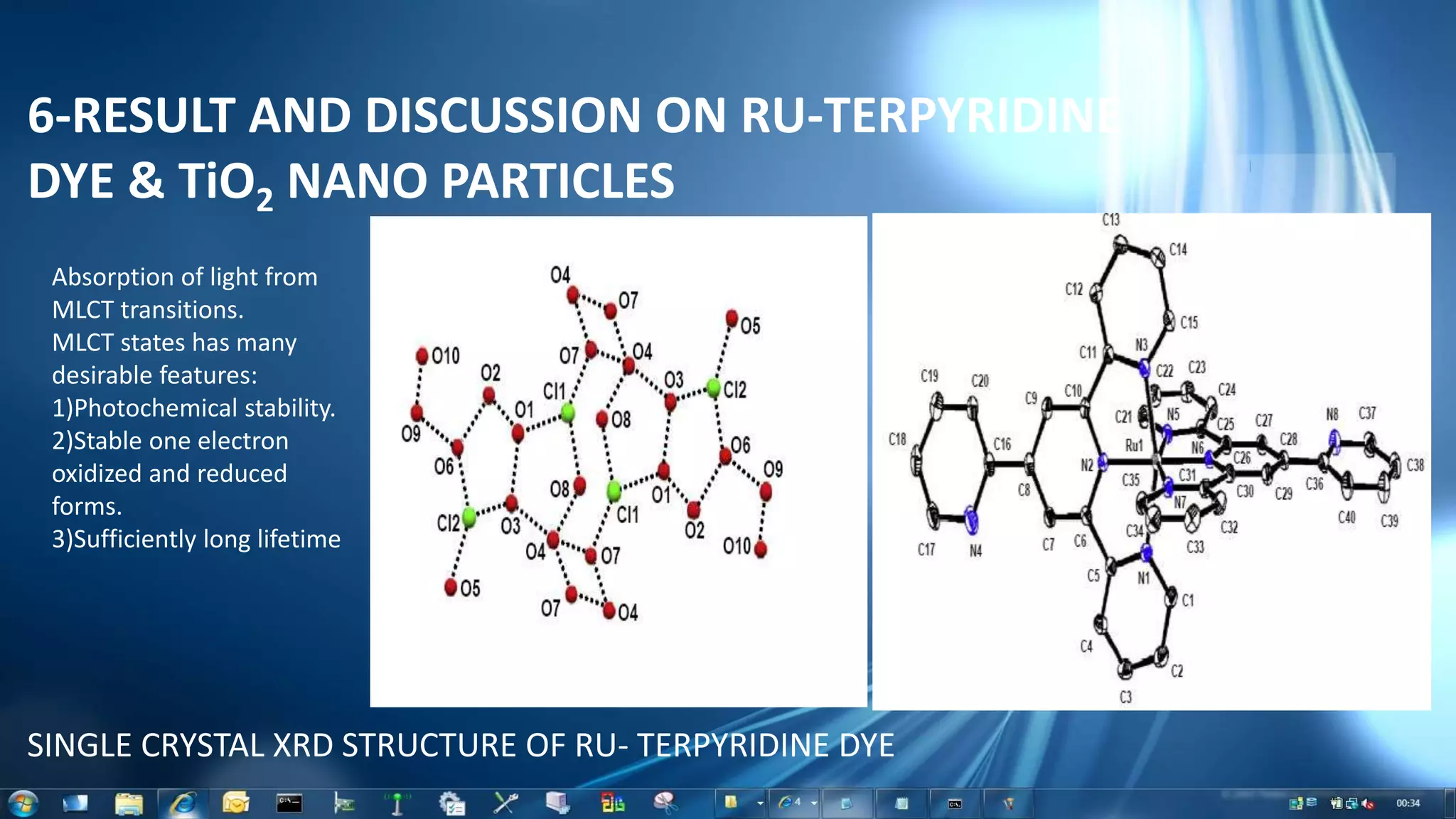
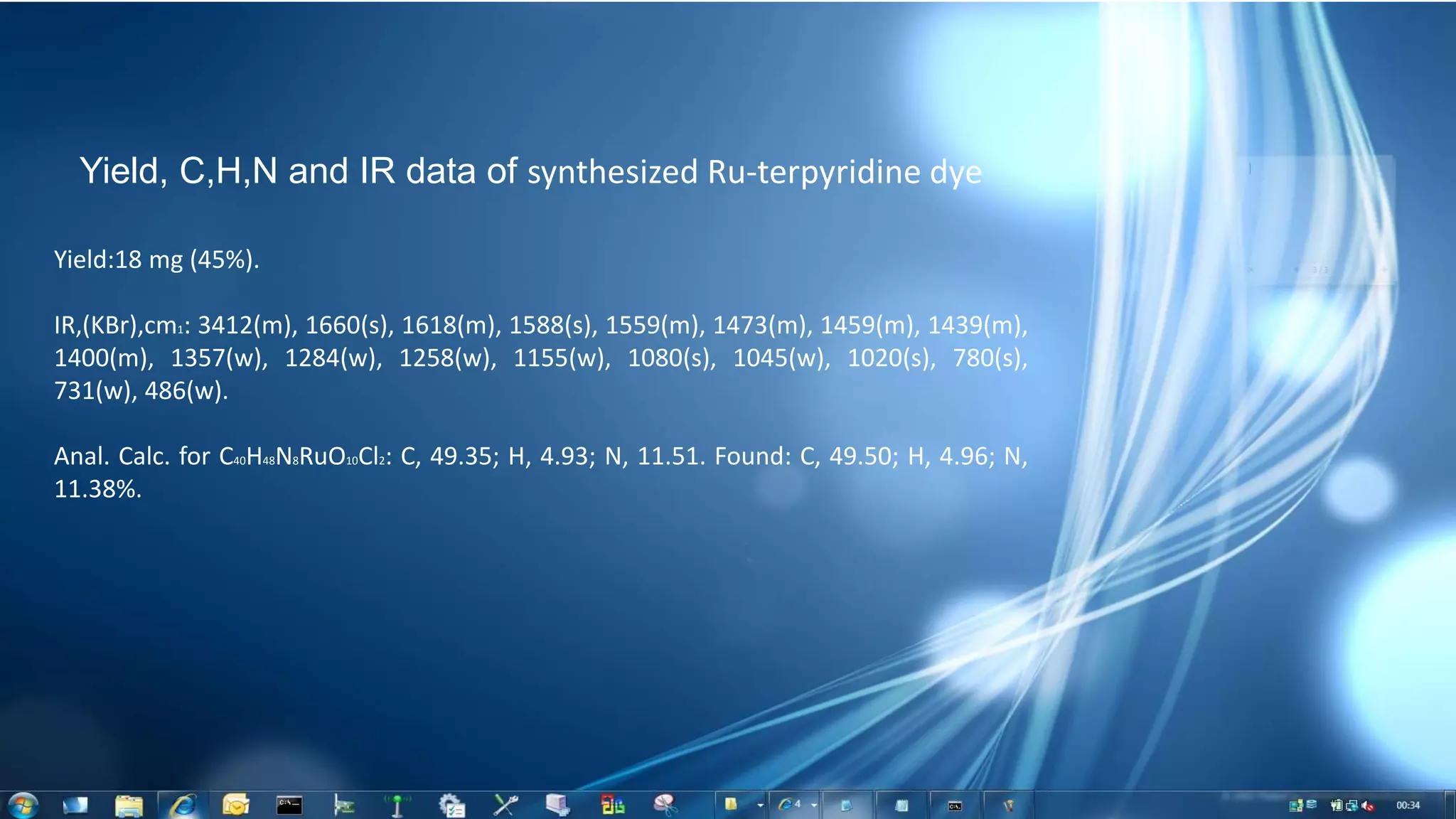
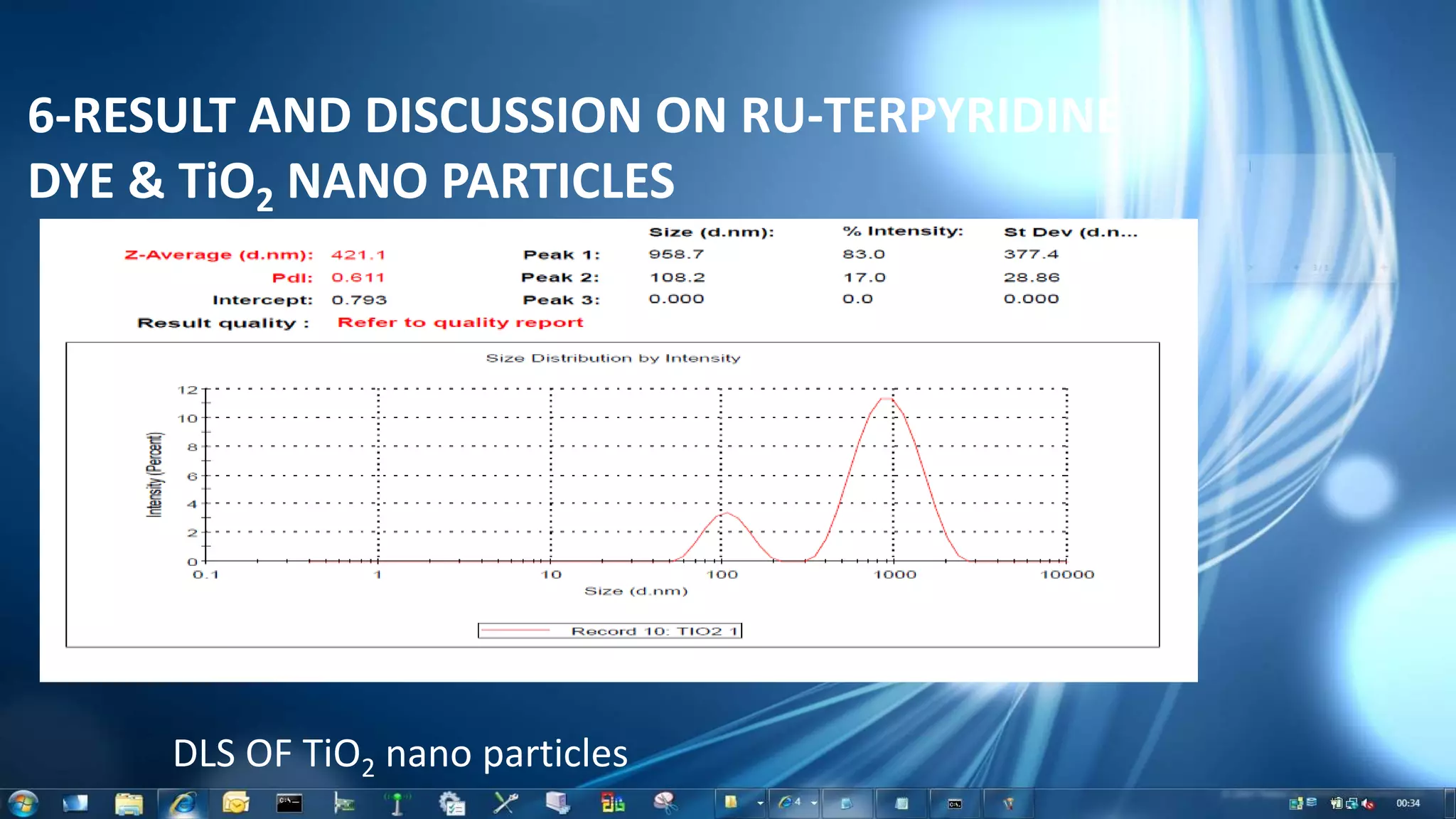
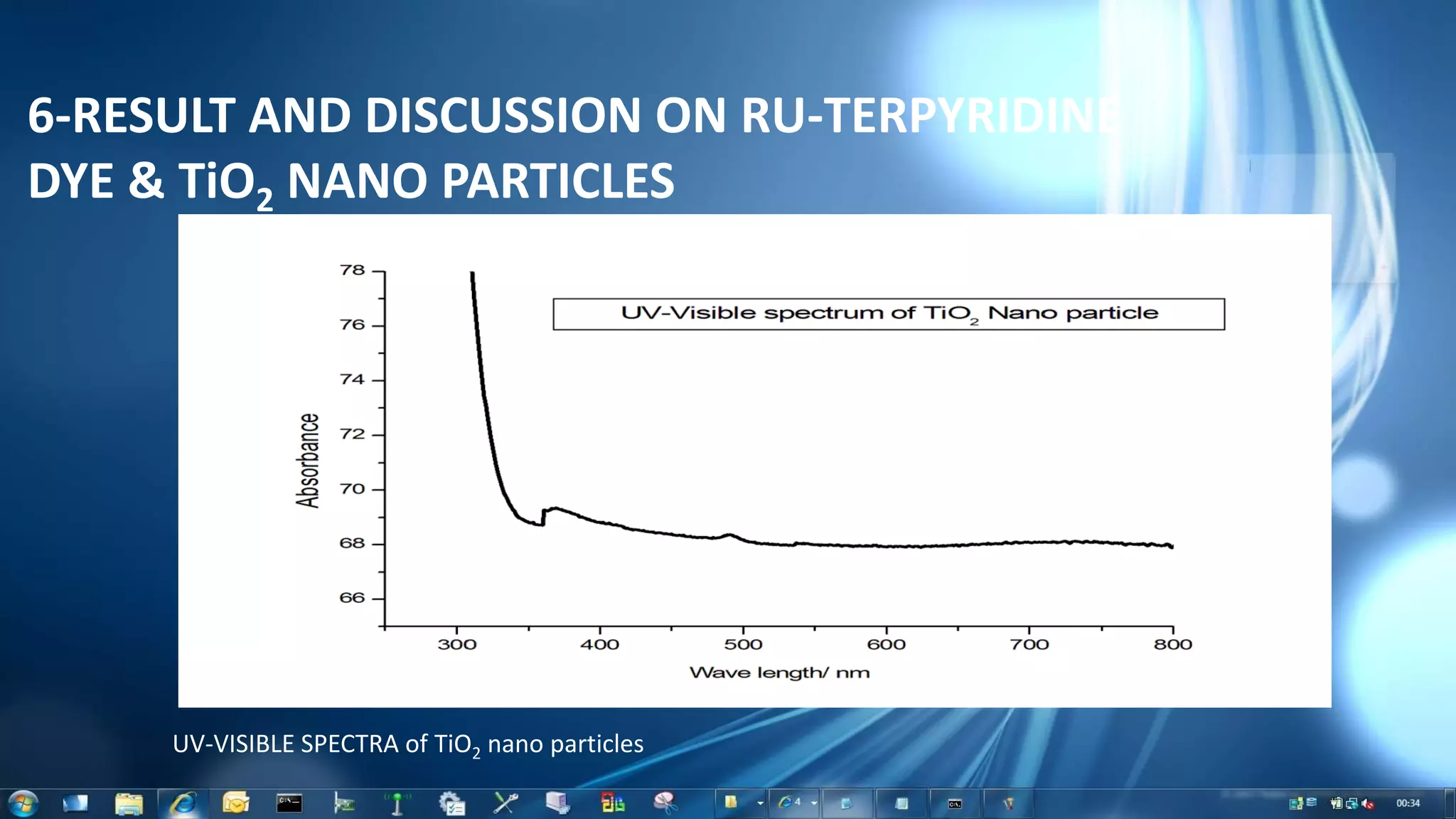
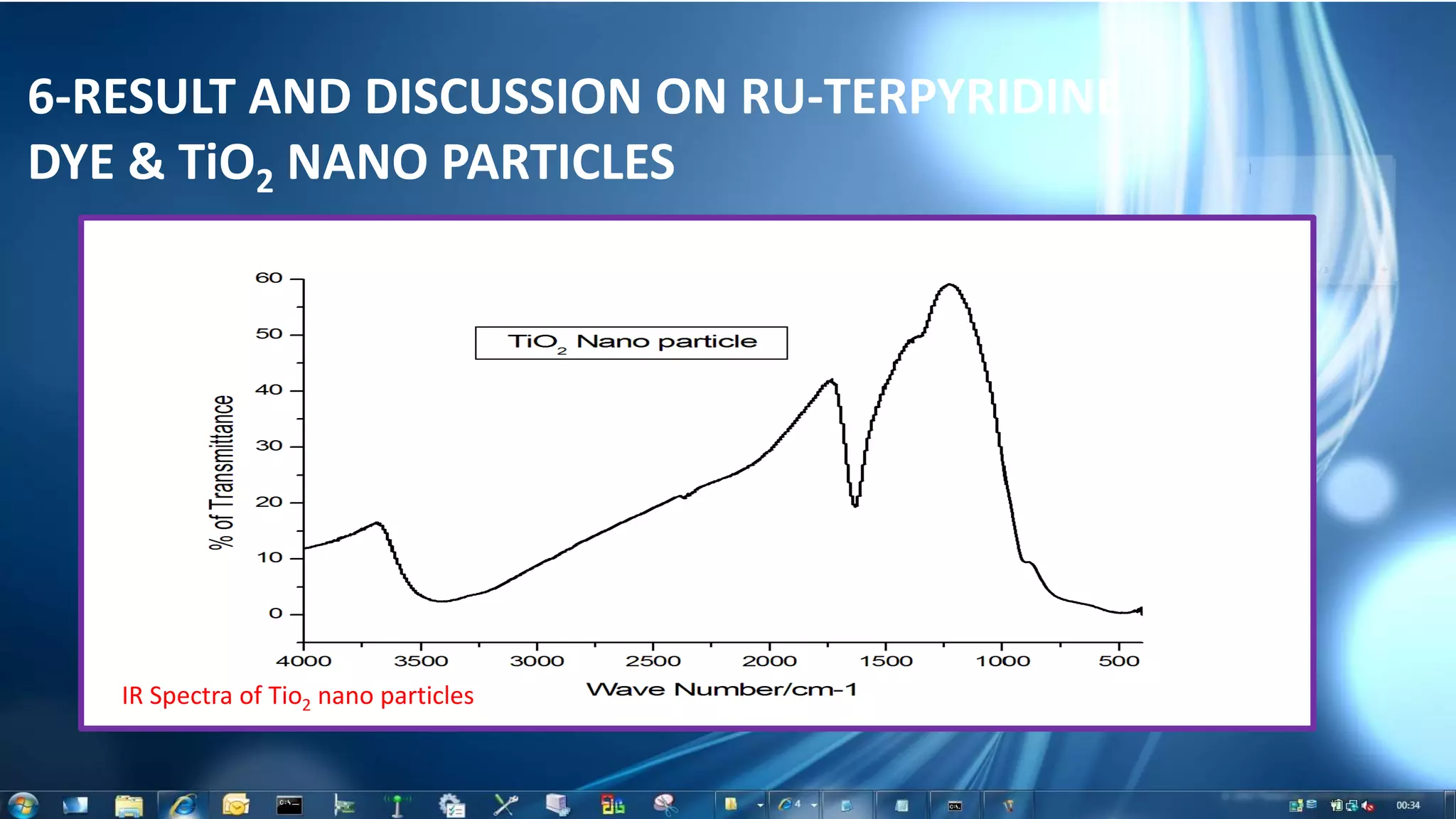
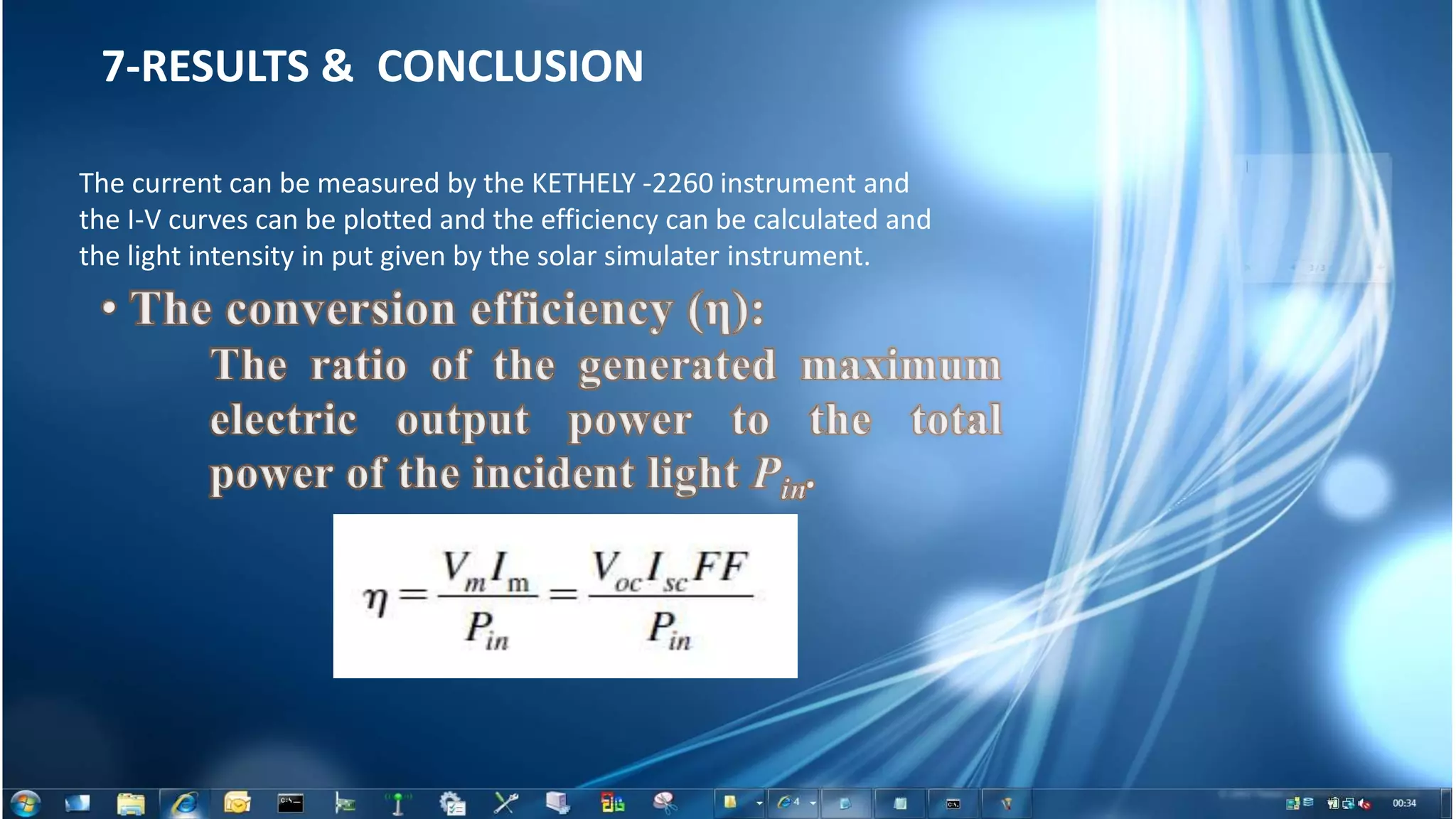
This document summarizes the synthesis and characterization of Ruthenium terpyridine complex and TiO2 nanoparticles for use in dye-sensitized solar cells (DSSCs). It describes the synthesis of the Ruthenium terpyridine dye through refluxing Ruthenium chloride with terpyridine. It also describes the hydrothermal synthesis of TiO2 nanoparticles and their characterization through techniques like DLS, UV-visible spectroscopy, and IR spectroscopy. Finally, it outlines the steps to fabricate a DSSC using the synthesized Ruthenium dye and TiO2 nanoparticles, and describes how performance would be evaluated by measuring current-voltage characteristics under light.