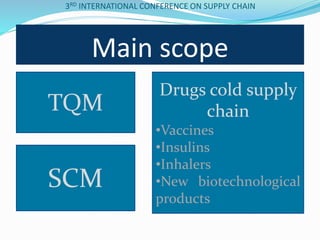
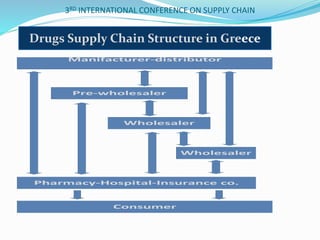
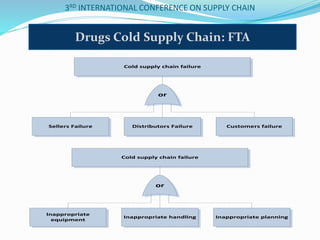
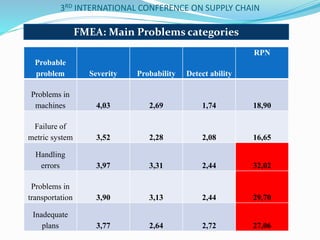
The document discusses implementing total quality management tools in cold drug supply chains. It focuses on detecting potential problems, understanding and evaluating them, and implementing corrective actions. The document outlines the structure of a study on Greece's drug supply chain, including analyzing operations and detecting faults. Research methodology included a questionnaire to pharmacists, wholesalers and companies. Data analysis identified problems like inappropriate refrigeration machines and handling errors. Failure mode and effects analysis identified top problems and their causes. Corrective actions proposed included clear procedures, communication, evaluation and employee involvement.