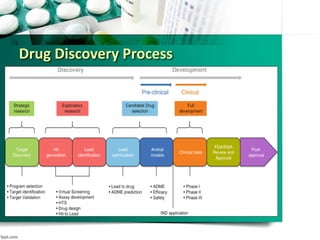
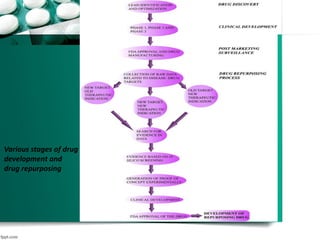
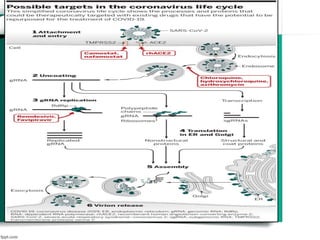
The document discusses drug repurposing as a method of finding new uses for existing drugs, which can significantly reduce development costs and timelines. It highlights various successful examples of repurposed drugs, such as thalidomide for multiple myeloma and celecoxib for colorectal cancer, and outlines the benefits of repurposing, including safety and lower risk. The conclusion emphasizes the potential of drug repurposing to enhance pharmaceutical product pipelines and provide new treatments for serious diseases.