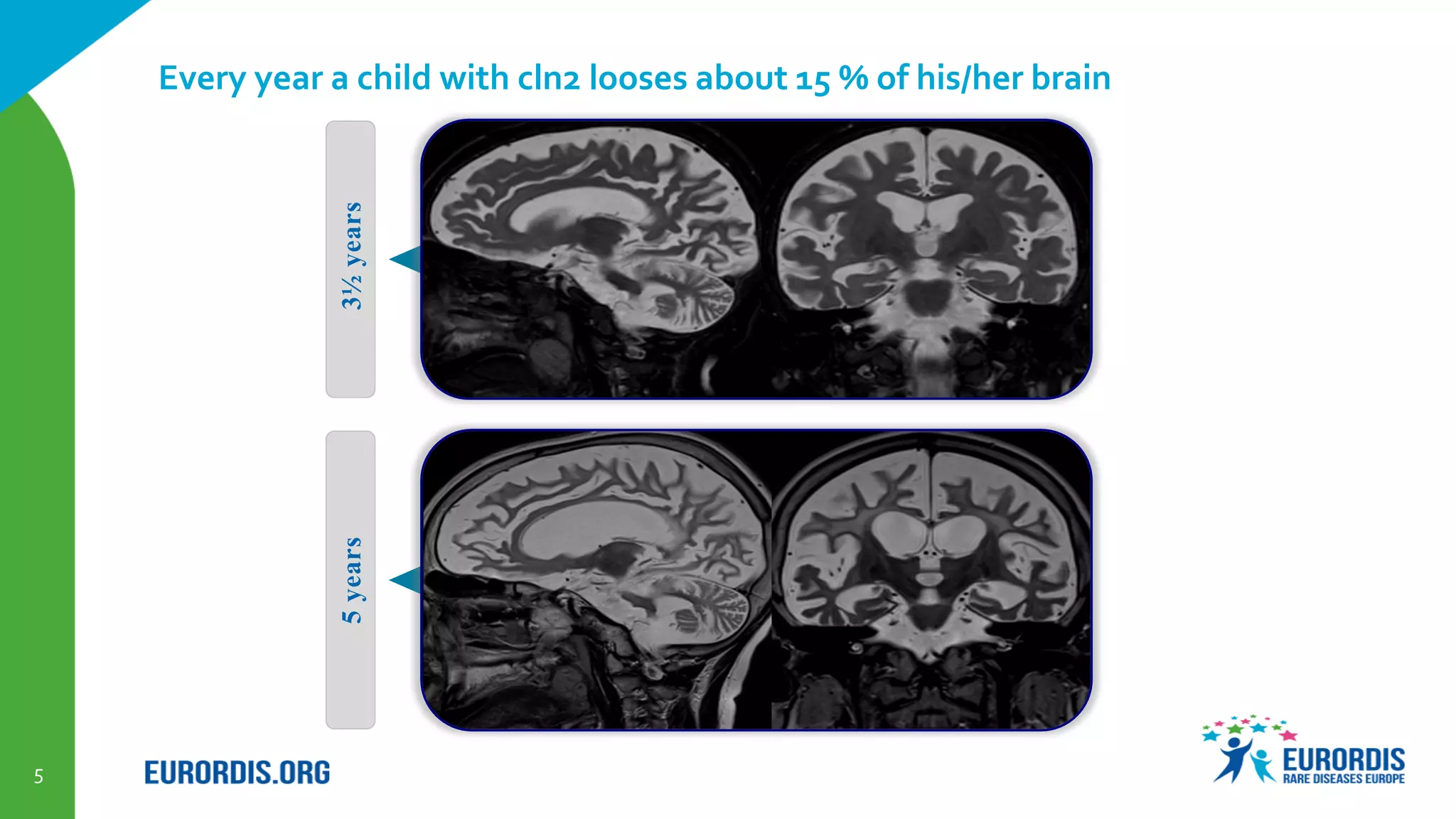
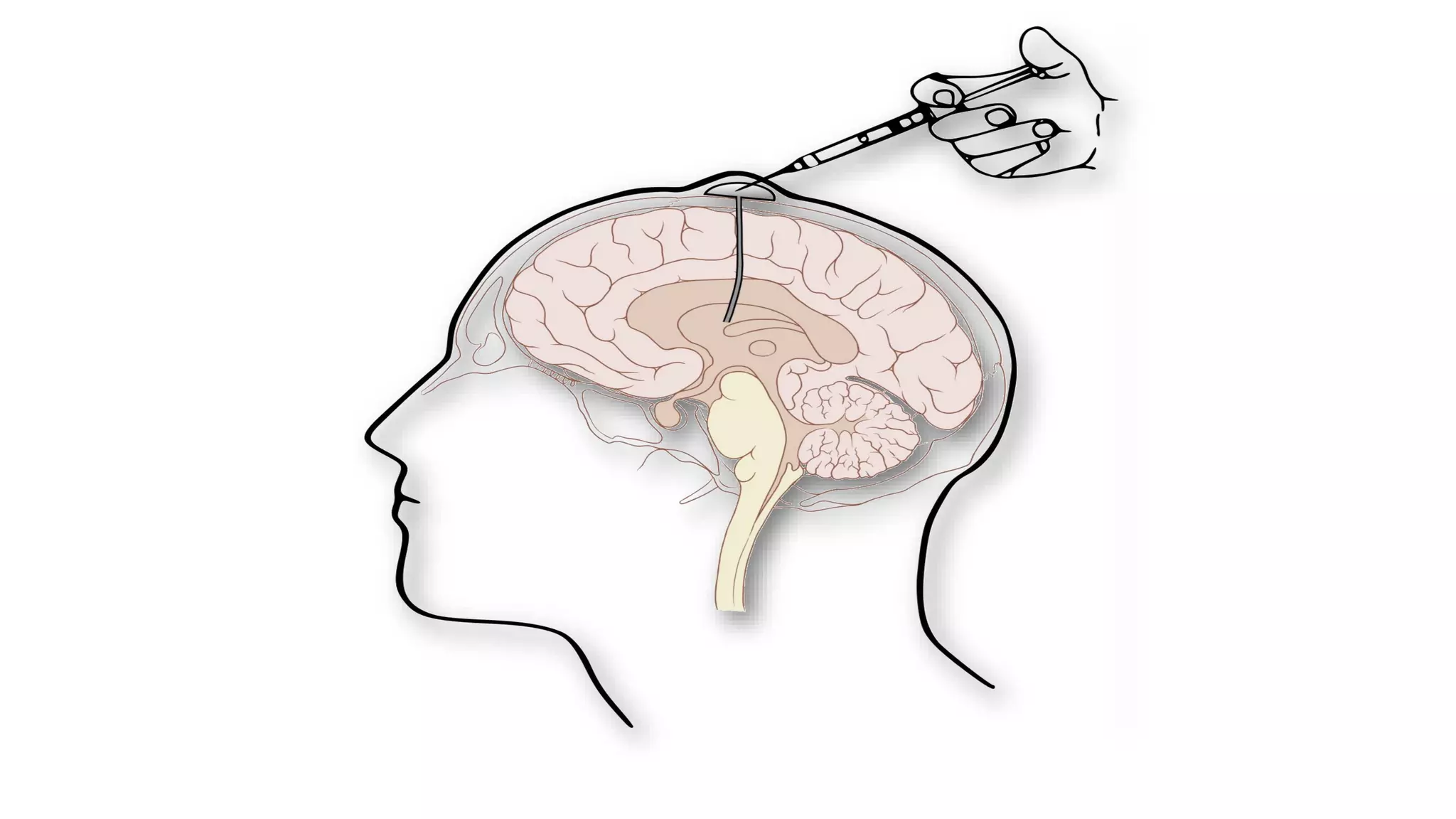
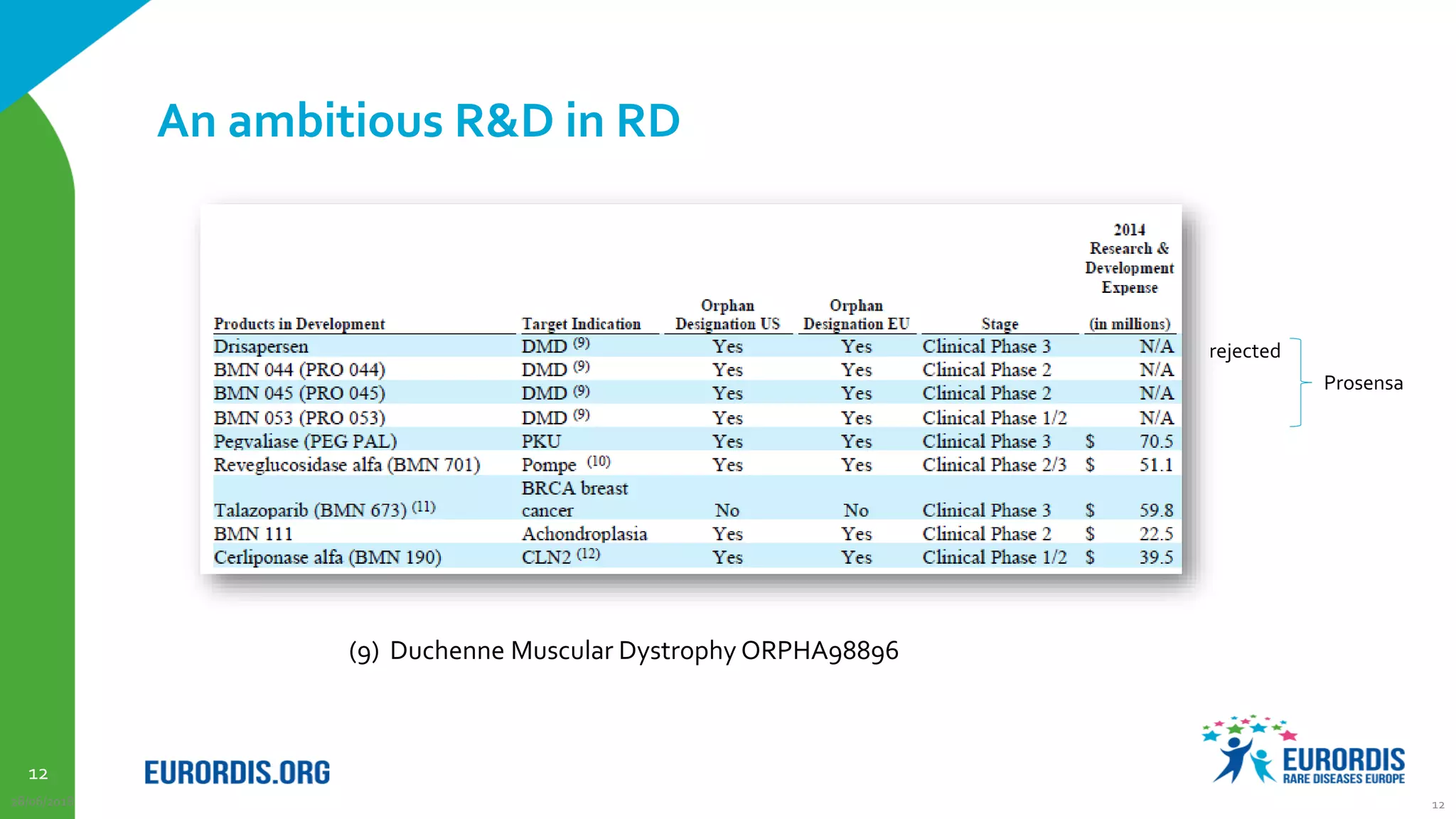
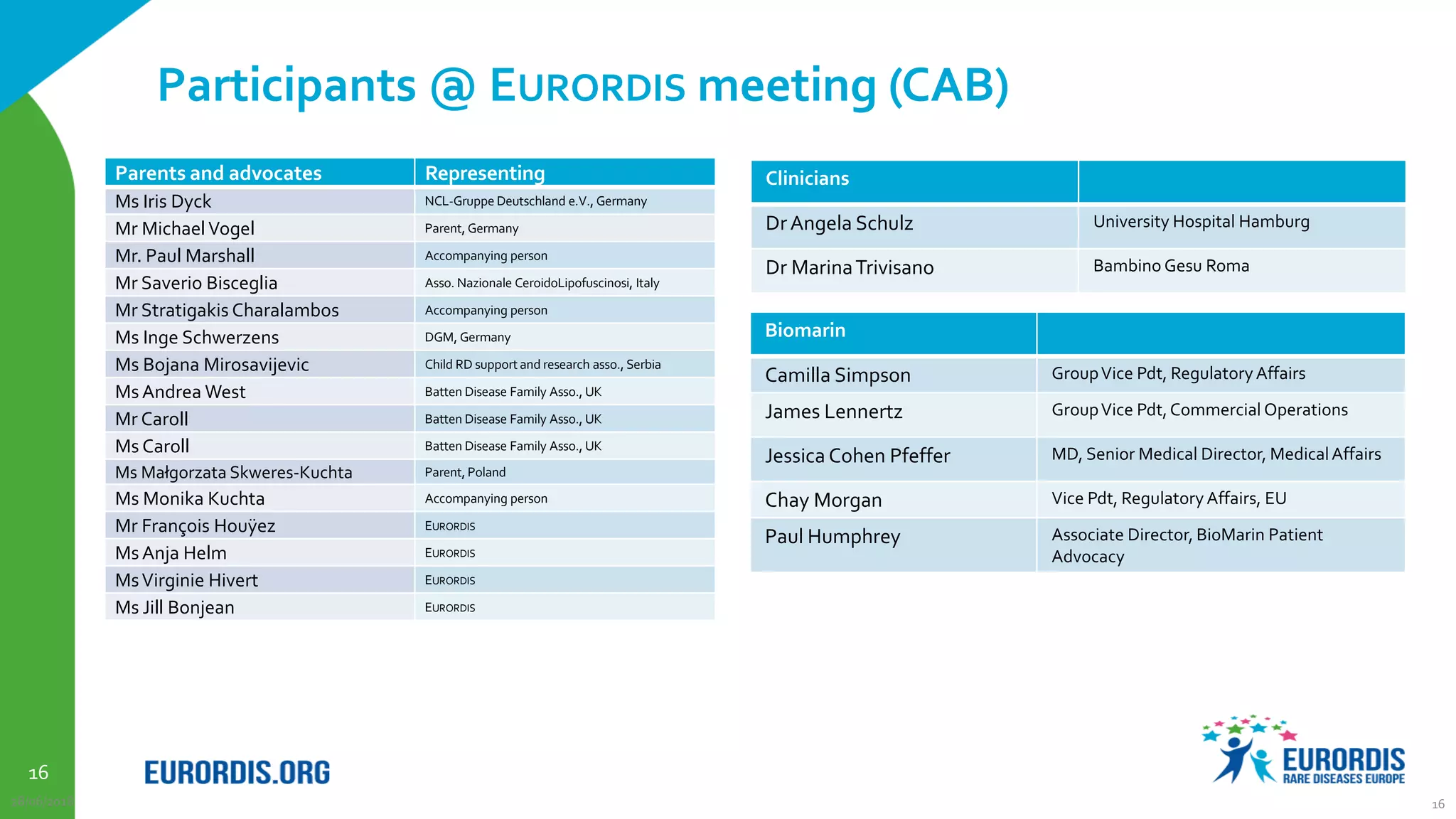
Compassionate use is a program that provides access to experimental drugs for patients with serious conditions who have no other treatment options. The document discusses compassionate use of BMN 190, an experimental drug for Batten disease. It notes the disease causes rapid neurological decline in children. The company BioMarin plans to submit the drug for approval in 2016 based on results from a small trial. It also plans compassionate use programs in Europe and the US if regulators provide positive feedback. However, some patients may not have access soon enough given the drug's challenges to produce and administer. The document outlines regulatory pathways and EURORDIS' recommendations to ensure compassionate use programs are run effectively.