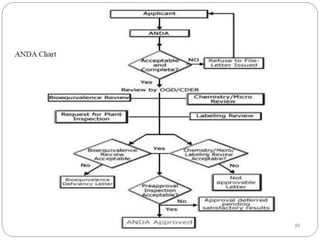
This document provides an overview of regulatory affairs processes for new drugs, including Investigational New Drug (IND) applications, New Drug Applications (NDA), and Abbreviated New Drug Applications (ANDA). It defines these terms and describes the required contents, format, review process and differences between IND, NDA, and ANDA submissions to regulatory agencies like the FDA. Key points covered include the types of clinical trials and data required for each application and how generic drugs can demonstrate bioequivalence rather than conducting new clinical trials in an ANDA.