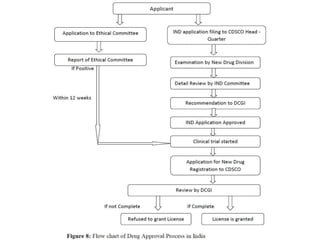
The document discusses the new drug approval process. It begins with introducing key terms like new drug and New Drug Application (NDA). It then outlines the objectives of an NDA which are to evaluate the drug's safety, effectiveness, proposed labeling, and manufacturing quality. The document proceeds to describe the two phases of new drug approval - clinical trials and marketing authorization. Key steps involve completing pre-clinical studies, submitting clinical trial applications, conducting Phase I-IV trials, and submitting the NDA which includes all trial data for regulatory review and approval. Ongoing monitoring is also needed after approval.