The document discusses several regulatory concepts and publications related to drug approval:
- The Orange Book identifies FDA-approved drug products and evaluates therapeutic equivalence of generic drugs. It aims to inform prescribing and is updated regularly.
- The Purple Book similarly lists licensed biological products, reference products, biosimilars, and exclusivity information.
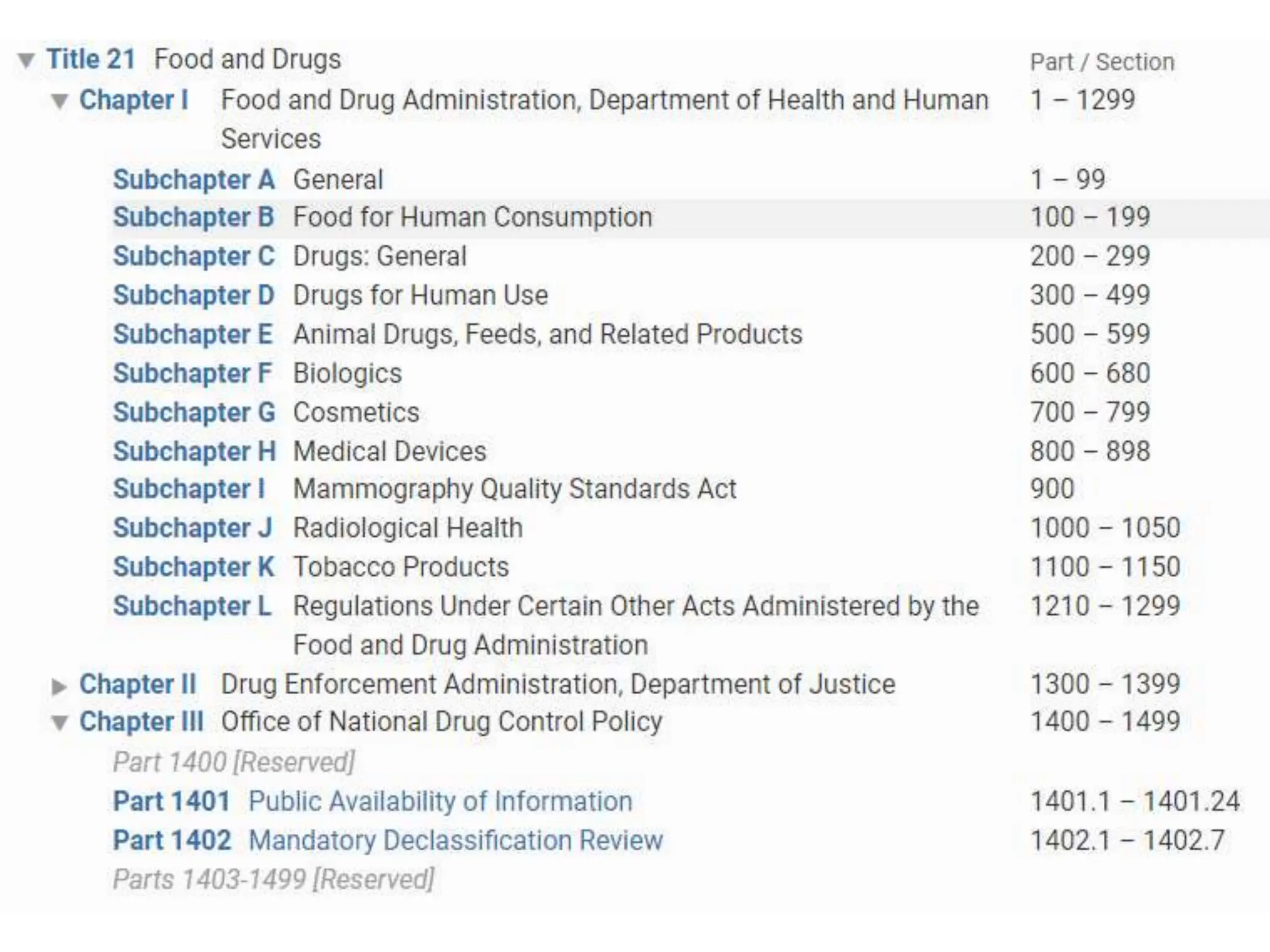
- The Federal Register and Code of Federal Regulations publish and codify rules and regulations from federal agencies like the FDA. Titles and chapters address specific regulatory areas like drugs and devices.






![Section 505(j)(8)(B) of the FD&C Act describes certain conditions under which a test drug and
reference listed drug shall be considered bioequivalent:
I)the rate and extent of absorption of the [test] drug do not show a significant difference from
the rate and extent of absorption of the [reference] listed drug when administered at the same
molar dose of the therapeutic ingredient under similar experimental conditions in either a single
dose or multiple doses; or
(ii)the extent of absorption of the [test] drug does not show a significant difference from the
extent of absorption of the[reference] listed drug when administered at the same molar dose of
the therapeutic ingredient under similar experimental conditions in either a single dose or
multiple doses](https://image.slidesharecdn.com/regulatoryconcepts-240210041348-5074f8b9/75/regulatory-concepts-endfkadflsdjfajfd-ppt-7-2048.jpg)