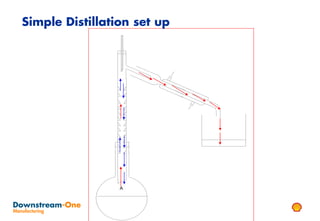
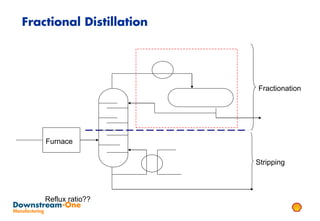
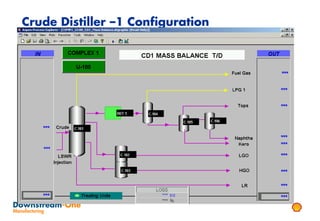
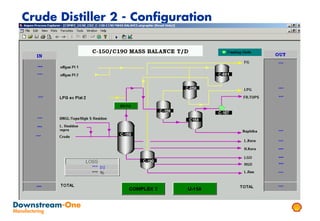
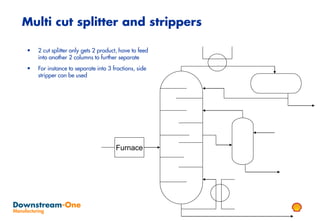
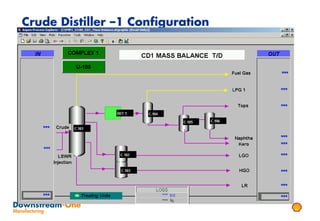
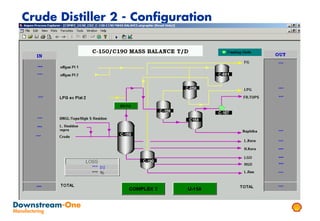
Distillation is the most common separation technique that involves evaporating and condensing components of a liquid mixture to separate them based on differences in their boiling points. It consumes large amounts of energy for heating and cooling. Fractional distillation uses a fractionating column and refluxing to improve separation efficiency compared to simple distillation. Various parameters like reflux ratio, pressure, temperature controls are optimized to achieve desired purities and specifications of fractions in a distillation column. Stripping steam is also used to help separate lighter components from heavier fractions.